Abstract
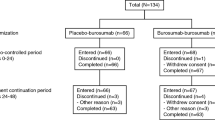
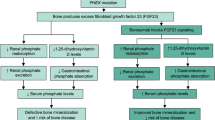
The anti-fibroblast growth factor 23 monoclonal antibody burosumab corrects hypophosphatemia in adults with X-linked hypophosphatemia (XLH) and improves pain, stiffness, physical function, and fatigue. This post hoc subgroup analysis used data from the 24-week placebo-controlled period of a phase 3 study in 134 adults with XLH (ClinicalTrials.gov NCT02526160), to assess whether the benefits of burosumab are evident in 14 clinically relevant subgroups defined by baseline demographic and functional criteria, including sex, Brief Pain Inventory-short form (BPI-SF) Average And Worst Pain, region, race, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC®) Stiffness, Physical Function and Pain domains and total score, use of opioid/other pain medication, active fractures/pseudo-fractures, and 6-min walk test distance. There were no statistically significant interactions between any of the subgroups and treatment arm for any endpoint. Higher proportions of subjects achieved mean serum phosphate concentration above the lower limit of normal (the primary endpoint) with burosumab than with placebo in all subgroups. For the key secondary endpoints (WOMAC Stiffness and Physical Function; BPI-SF Worst Pain) individual subgroup categories showed improvements with burosumab relative to placebo. For additional efficacy endpoints, burosumab was favored in some subgroups but differences were not significant and confidence intervals were wide. For some endpoints the treatment effect is small at 24 weeks in all subjects. This subgroup analysis shows that burosumab was largely superior to placebo across endpoints in the 14 clinically relevant subgroup variables at 24 weeks and is likely to benefit all symptomatic adults with active XLH.




Similar content being viewed by others
Data Availability
The corresponding author had full access to all the data in the study.
References
Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL (2011) A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res 26(7):1381–1388. https://doi.org/10.1002/jbmr.340
Chesher D, Oddy M, Darbar U, Sayal P, Casey A, Ryan A, Sechi A et al (2018) Outcome of adult patients with X-linked hypophosphatemia caused by PHEX gene mutations. J Inherit Metab Dis 41(5):865–876. https://doi.org/10.1007/s10545-018-0147-6
Hardy DC, Murphy WA, Siegel BA, Reid IR, Whyte MP (1989) X-linked hypophosphatemia in adults: prevalence of skeletal radiographic and scintigraphic features. Radiology 171(2):403–414. https://doi.org/10.1148/radiology.171.2.2539609
Reid IR, Hardy DC, Murphy WA, Teitelbaum SL, Bergfeld MA, Whyte MP (1989) X-linked hypophosphatemia: a clinical, biochemical, and histopathologic assessment of morbidity in adults. Medicine (Baltimore) 68(6):336–352
Skrinar A, Dvorak-Ewell M, Evins A, Macica C, Linglart A, Imel EA, Theodore-Oklota C et al (2019) The lifelong impact of X-linked hypophosphatemia: results from a burden of disease survey. J Endocr Soc 3(7):1321–1334. https://doi.org/10.1210/js.2018-00365
Ferizovic N, Marshall J, Williams AE, Mughal MZ, Shaw N, Mak C, Gardiner O et al (2020) Exploring the burden of X-linked hypophosphataemia: an opportunistic qualitative study of patient statements generated during a technology appraisal. Adv Ther 37(2):770–784. https://doi.org/10.1007/s12325-019-01193-0
Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D, Wicart P et al (2019) Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol 15(7):435–455. https://doi.org/10.1038/s41581-019-0152-5
Insogna KL, Briot K, Imel EA, Kamenicky P, Ruppe MD, Portale AA, Weber T et al (2018) A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res 33(8):1383–1393. https://doi.org/10.1002/jbmr.3475
Portale AA, Carpenter TO, Brandi ML, Briot K, Cheong HI, Cohen-Solal M, Crowley R et al (2019) Continued beneficial effects of burosumab in adults with X-linked hypophosphatemia: results from a 24-week treatment continuation period after a 24-week double-blind placebo-controlled period. Calcif Tissue Int 105(3):271–284. https://doi.org/10.1007/s00223-019-00568-3
Imel EA, Zhang X, Ruppe MD, Weber TJ, Klausner MA, Ito T, Vergeire M et al (2015) Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab 100(7):2565–2573. https://doi.org/10.1210/jc.2015-1551
Briot K, Portale AA, Brandi ML, Carpenter TO, Cheong HI, Cohen-Solal M, Crowley RK et al (2021) Burosumab treatment in adults with X-linked hypophosphataemia: 96-week patient-reported outcomes and ambulatory function from a randomised phase 3 trial and open-label extension. RMD Open. https://doi.org/10.1136/rmdopen-2021-001714
Cleeland CS (2009) The Brief Pain Inventory User Guide. https://www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/BPI_UserGuide.pdf
Skrinar A, Theodore-Oklota C, Bonner N, Arbuckle R, Williams A, Nixon A (eds) (2019) Confirmatory psychometric validation of the Brief Pain Inventory (BPI-SF) in adult X-linked hypophosphatemia (XLH). ISPOR; Copenhagen
Bellamy N (2012) WOMAC osteoarthritis index user guide. Version X. . Brisbance
Skrinar A, Theodore-Oklota C, Bonner N, Arbuckle R, Williams A, Nixon A (2019) Confirmatory psychometric validation of the Western Ontario and McMaster Universities Osteoarthritis Inventory (WOMAC) in adult X-linked hypophosphatemia (XLH). Value Health 22(suppl 3):S870
Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85(5):1186–1196
Nixon A, Williams A, Theodore-Oklota C (eds) Psychometric validation of the Brief Fatigue Inventory (BFI) in adult x-linked hypophosphatemia (XLH). ISPOR; 2020; Orlando
ATS. ATS Statement: Guidelines for the Six-Minute Walk Test 2002. https://www.thoracic.org/statements/resources/pfet/sixminute.pdf
Javaid MK, Ward L, Pinedo-Villanueva R, Rylands AJ, Williams A, Insogna K, Imel EA (2021) Musculoskeletal features in adults with X-linked hypophosphatemia: an analysis of clinical trial and survey data. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgab739
Davis CE, Leffingwell DP (1990) Empirical Bayes estimates of subgroup effects in clinical trials. Control Clin Trials 11(1):37–42. https://doi.org/10.1016/0197-2456(90)90030-6
Acknowledgements
The authors thank the participants, caregivers, investigators, and healthcare professionals who participated in this study. The authors would like to thank Mark Nixon PhD and Jennifer Liu PhD for support with quality checking and interpretation of the statistical analyses, Helen Barham PhD (thetextdoctor) for writing and editing support, and Jenna Zan of Zed for creating the figures.
Funding
This study was funded by Ultragenyx Pharmaceutical Inc. and Kyowa Kirin International.
Author information
Authors and Affiliations
Contributions
Study design: KI, EAI, AP, and TC; data collection: MLB, SJB, KB, TC, HIC, MC-S, RKC, RE, YI, EAI, KI, SWI, NI, KJ, PK, RK, TK, RHL, FP, PP, AP, SHR, HT, TW, H-WY, and YT; data analysis: WS, data interpretation: all authors, drafting of the manuscript: MLB, AN, AW, and WS; revising manuscript content: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The following authors served as clinical investigators for one or more studies, including this trial, sponsored by Ultragenyx Pharmaceutical Inc. in partnership with Kyowa Kirin International plc: MLB, SJdB, KB, TOC, HIC, MC-S, RKC, RE, YI, EAI, KI, SI, NI, MKJ, PK, RK, TK, RHL, FP, PP, AAP, SHR, HT, TJW, H-WY, YT. SJdB, TOC, EAI, RHL, FP, AAP, and TJW have also received honoraria from Ultragenyx Pharmaceutical Inc. for serving as an advisory board member or for delivering lectures and AAP and TJW have received travel reimbursement. TOC, YI, MJK, RK, TK, and YT have received honoraria for serving as an advisory board member or for speaker fees from Kyowa Kirin International plc. During the past 3 years, the following authors have received grants outside of the submitted work: RE from Alexion Pharmaceuticals, Amgen, Immunodiagnostic Systems Holdings plc, Nittobo Medical Co., Ltd., and Roche; SI from Alexion Pharmaceuticals, Radius Health, and Takeda Pharmaceutical Company; NI, MJK, and RHL from Kyowa Kirin International plc; TK from Teijin Pharma; PP from Ultragenyx Pharmaceutical Inc.; and SHR from Amgen, UCB, and AstraZeneca. PP is currently an employee of Ascendis Pharma Inc. AW is an employee of Kyowa Kirin International plc and WS is an employee of Kyowa Kirin Pharmaceutical Development, Inc. AN and MN are employees of Chilli Consultancy and have received consultancy fees from Kyowa Kirin International plc to support the development of this manuscript and for projects outside this submitted work.
Human and Animal Rights and Informed Consent statements
As stated in the abstract, this study is a post hoc subgroup analysis from an approved phase 3 Clinical Trial (ClinicalTrials.gov NCT02526160) for which informed consent was obtained from all participants in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brandi, M.L., Jan de Beur, S., Briot, K. et al. Efficacy of Burosumab in Adults with X-linked Hypophosphatemia (XLH): A Post Hoc Subgroup Analysis of a Randomized Double-Blind Placebo-Controlled Phase 3 Study. Calcif Tissue Int 111, 409–418 (2022). https://doi.org/10.1007/s00223-022-01006-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01006-7