Abstract
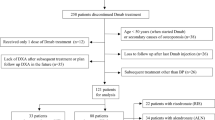
Discontinuation of denosumab (DMab) is associated with decline in bone density. Whether raloxifene can be effective to attenuate bone loss after DMab discontinuation in certain conditions when other antiresorptives cannot be used remains unclear. Data on postmenopausal women with osteoporosis who discontinued DMab treatment after short-term use (1-to-4 doses) at Severance Hospital, Seoul, Korea, between 2017 and 2021 were reviewed. Changes in bone mineral density (BMD) at 12 months after DMab discontinuation was compared between sequential raloxifene users (DR) and those without any sequential antiresorptive (DD) after 1:1 propensity score matching. In matched cohort (66 patients; DR n = 33 vs. DD n = 33), mean age (69.3 ± 8.2 years) and T-score (lumbar spine − 2.2 ± 0.7; total hip − 1.6 ± 0.6) did not differ between two groups at the time of DMab discontinuation. Sequential treatment to raloxifene in DR group attenuated the bone loss in lumbar spine after DMab discontinuation compared to DD group (DR vs. DD; − 2.8% vs. − 5.8%, p = 0.013). The effect of raloxifene on lumbar spine BMD changes remained robust (adjusted β + 2.92 vs. DD, p = 0.009) after adjustment for covariates. BMD loss at femoral neck (− 1.70% vs. − 2.77%, p = 0.673) and total hip (− 1.42% vs. − 1.44%, p = 0.992) did not differ between two groups. Compared to BMD at DMab initiation, DR partially retained BMD gain by DMab treatment in lumbar spine (+ 3.7%, p = 0.003) and femoral neck (+ 2.8%, p = 0.010), whereas DD did not. Raloxifene use after DMab treatment attenuated lumbar spine BMD loss in postmenopausal women with short exposures (< 2 years) to DMab.



Similar content being viewed by others
References
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, FREEDOM Trial (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwinski E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S (2017) 10 Years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5:513–523
Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D (2019) Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab 104:1595–1622
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB (2020) American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr Pract 26:1–46
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP (2018) Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res 33:190–198
Grassi G, Chiodini I, Palmieri S, Cairoli E, Arosio M, Eller-Vainicher C (2021) Bisphosphonates after denosumab withdrawal reduce the vertebral fractures incidence. Eur J Endocrinol 185:387–396
Sølling AS, Harsløf T, Langdahl B (2021) Treatment with zoledronate subsequent to denosumab in osteoporosis: a 2-year randomized study. J Bone Miner Res 36:1245–1254
Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Makras P (2019) Zoledronate for the prevention of bone loss in women discontinuing denosumab treatment. A prospective 2-year clinical trial. J Bone Miner Res 34:2220–2228
Kadaru T, Shibli-Rahhal A (2021) Zoledronic acid after treatment with denosumab is associated with bone loss within 1 year. J Bone Metab 28:51–58
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Glüer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645
Michalská D, Stepan JJ, Basson BR, Pavo I (2006) The effect of raloxifene after discontinuation of long-term alendronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 91:870–877
Ebina K, Hashimoto J, Kashii M, Hirao M, Miyama A, Nakaya H, Tsuji S, Takahi K, Tsuboi H, Okamura G, Etani Y, Takami K, Yoshikawa H (2021) Effects of follow-on therapy after denosumab discontinuation in patients with postmenopausal osteoporosis. Mod Rheumatol 31:485–492
Hanson J (1997) Standardization of femur BMD. J Bone Miner Res 12:1316–1317
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43:222–229
Kendler D, Chines A, Clark P, Ebeling PR, McClung M, Rhee Y, Huang S, Stad RK (2020) Bone mineral density after transitioning from denosumab to alendronate. J Clin Endocrinol Metab 105:e255-264
Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T (2020) A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res 35:1207–1215
Tsourdi E, Zillikens MC, Meier C, Body JJ, Gonzalez Rodriguez E, Anastasilakis AD, Abrahamsen B, McCloskey E, Hofbauer LC, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Pepe J, Palermo A, Langdahl B (2020) Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa756
Makras P, Appelman-Dijkstra NM, Papapoulos SE, van Wissen S, Winter EM, Polyzos SA, Yavropoulou MP, Anastasilakis AD (2021) The duration of denosumab treatment and the efficacy of zoledronate to preserve bone mineral density after its discontinuation. J Clin Endocrinol Metab 106:e4155–e4162
Seeman E, Crans GG, Diez-Perez A, Pinette KV, Delmas PD (2006) Anti-vertebral fracture efficacy of raloxifene: a meta-analysis. Osteoporos Int 17:313–316
Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, Almasri J, Farah W, Sarigianni M, Muthusamy K, Al Nofal A, Haydour Q, Wang Z, Murad MH (2019) Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab 104:1623–1630
Allen MR, Hogan HA, Hobbs WA, Koivuniemi AS, Koivuniemi MC, Burr DB (2007) Raloxifene enhances material-level mechanical properties of femoral cortical and trabecular bone. Endocrinology 148:3908–3913
Crandall CJ, Newberry SJ, Diamant A, Lim YW, Gellad WF, Booth MJ, Motala A, Shekelle PG (2014) Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med 161:711–723
Chiu WY, Chien JY, Yang WS, Juang JM, Lee JJ, Tsai KS (2014) The risk of osteonecrosis of the jaws in Taiwanese osteoporotic patients treated with oral alendronate or raloxifene. J Clin Endocrinol Metab 99:2729–2735
Lamy O, Stoll D, Aubry-Rozier B, Rodriguez EG (2019) Stopping denosumab. Curr Osteoporos Rep 17:8–15
Boquete-Castro A, Gómez-Moreno G, Calvo-Guirado JL, Aguilar-Salvatierra A, Delgado-Ruiz RA (2016) Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin Oral Implants Res 27:367–375
Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Howe TS, van der Meulen MC, Weinstein RS, Whyte MP (2014) Atypical subtrochanteric and diaphyseal femoral fractures: Second Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res 29:1–23
Burckhardt P, Faouzi M, Buclin T, Lamy O (2021) Fractures after denosumab discontinuation: a retrospective study of 797 cases. J Bone Miner Res 36:1717–1728
Swart KMA, van Vilsteren M, van Hout W, Draak E, van der Zwaard BC, van der Horst HE, Hugtenburg JG, Elders PJM (2018) Factors related to intentional non-initiation of bisphosphonate treatment in patients with a high fracture risk in primary care: a qualitative study. BMC Fam Pract 19:141
Kim BK, Kim CH, Min YK (2021) Preventing rebound-associated fractures after discontinuation of denosumab therapy: a Position Statement from the Health Insurance Committee of the Korean Endocrine Society. Endocrinol Metab (Seoul) 36:909–911
McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM (2017) Observations following discontinuation of long-term denosumab therapy. Osteoporos Int 28:1723–1732
Tripto-Shkolnik L, Fund N, Rouach V, Chodick G, Shalev V, Goldshtein I (2020) Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone 130:115150
Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A (2021) Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med 10:152
Gonzalez-Rodriguez E, Stoll D, Lamy O (2018) Raloxifene has no efficacy in reducing the high bone turnover and the risk of spontaneous vertebral fractures after denosumab discontinuation. Case Rep Rheumatol 2018:5432751
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107
Sainani KL (2012) Propensity scores: uses and limitations. PM R 4:693–697
Acknowledgements
This study was supported by the ‘SENTINEL (Severance ENdocrinology daTa scIeNcE pLatform)’ Program funded by the 2020 Research fund of Department of Internal Medicine, Severance Hospital, Seoul, Korea, and Sung-Kil Lim Research Award (4-2018-1215; DUCD000002) in statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Namki Hong, Sungjae Shin, Seunghyun Lee, Kyoung Jin Kim, and Yumie Rhee have no relevant financial or non-financial interests to disclose.
Statement of Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
This study was approved by Institutional Review Board of Severance Hospital, with a waiver of written consent since the study design was based on retrospective medical record review (IRB No. 4-2021-1346).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hong, N., Shin, S., Lee, S. et al. Raloxifene Use After Denosumab Discontinuation Partially Attenuates Bone Loss in the Lumbar Spine in Postmenopausal Osteoporosis. Calcif Tissue Int 111, 47–55 (2022). https://doi.org/10.1007/s00223-022-00962-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-00962-4