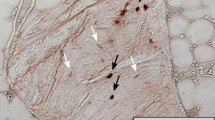
Abstract
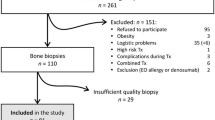
Chronic kidney disease-mineral and bone disorder has been associated with increasing morbid-mortality. The aim of this study was to determine the prevalence and phenotype of bone disease before transplantation and to correlate FGF23 and sclerostin levels with bone histomorphometry, and study possible associations between FGF23, sclerostin, and bone histomorphometry with cardiovascular disease and mortality. We performed a cross-sectional cohort study of a sample of 84 patients submitted to renal transplant, which were prospectively followed for 12 months. Demographic, clinical, and echocardiographic data were collected, laboratory evaluation, bone biopsy, and X-ray of the pelvis and hands were performed. Patient and graft survival were recorded. We diagnosed low bone turnover in 16 patients (19.5%); high bone turnover in 22 patients (26.8%); osteomalacia in 1 patient (1.2%), and mixed renal osteodystrophy in 3 patients (3.7%). At the end of 12 months, 5 patients had graft failure (5.9%), 4 had a cardiovascular event (4.8%), and 4 died. Age was associated with low remodeling disease, whereas high BALP and phosphorus and low sclerostin with high turnover disease. Sclerostin was a risk factor for isolated low bone volume. High BALP, low phosphorus, and low FGF23 were risk factors for abnormal mineralization. FGF23 appears as an independent factor for severity of vascular calcifications and for cardiovascular events, whereas the presence of valve calcifications was associated with low volume and with turnover deviations. Sclerostin was associated a higher HR for death. Sclerostin and FGF23 seemed to provide higher cardiovascular risk, as well as low bone volume, which associated with extra-osseous calcifications.

Similar content being viewed by others
Data Availability
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Wolfe RA, Ashby VB, Milford EL et al (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341(23):1725–1730
Vervloet MG, Massy ZA, Brandenburg VM et al (2014) Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol 2(5):427–436
Evenepoel P, Rodriguez M, Ketteler M (2014) Laboratory abnormalities in CKD-MBD: markers, predictors, or mediators of disease? Semin Nephrol 34(2):151–163
Moe S, Drueke T, Cunningham J et al (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 69(11):1945–1953
Alshayeb HM, Josephson MA, Sprague SM (2013) CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis 61(2):310–325
Barreto FC, Barreto DV, Moyses RM et al (2008) K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int 73(6):771–777
Lamb EJ, Delaney MP (2014) Does PTH offer additive value to ALP measurement in assessing CKD-MBD? Perit Dial Int 34(7):687–691
Covic A, Voroneanu L, Apetrii M (2016) PTH and/or bone histology: are we still waiting for a verdict from the CKD-MBD grand jury? Am J Kidney Dis 67(4):535–538
Sprague SM, Bellorin-Font E, Jorgetti V et al (2016) Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis 67(4):559–566
Brandenburg VM, D’Haese P, Deck A et al (2016) From skeletal to cardiovascular disease in 12 steps-the evolution of sclerostin as a major player in CKD-MBD. Pediatr Nephrol 31(2):195–206
Brandenburg VM, Kramann R, Koos R et al (2013) Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol 14:219
Gutierrez OM, Mannstadt M, Isakova T et al (2008) Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359(6):584–592
Moyses RM, Schiavi SC (2015) Sclerostin, osteocytes, and chronic kidney disease - mineral bone disorder. Semin Dial 28(6):578–586
Adragao T, Pires A, Lucas C et al (2004) A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant 19(6):1480–1488
Dempster DW, Compston JE, Drezner MK et al (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):2–17
Neto R, Pereira L, Magalhães J et al (2021) SCLEROSTIN AND DKK1 CIRCULATING LEVELS ASSOCIATE WITH LOW BONE TURNOVER IN PATIENTS WITH CHRONIC KIDNEY DISEASE STAGES 3 AND 4. Clin Kidney J. https://doi.org/10.1093/ckj/sfab081
Jean G, Chazot C (2013) Sclerostin in CKD-MBD: one more paradoxical bone protein? Nephrol Dial Transplant 28(12):2932–2935
Cejka D, Marculescu R, Kozakowski N et al (2014) Renal elimination of sclerostin increases with declining kidney function. J Clin Endocrinol Metab 99(1):248–255
Roforth MM, Fujita K, McGregor UI et al (2014) Effects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. Bone 59:1–6
Zhu D, Mackenzie NC, Millan JL, Farquharson C, MacRae VE (2011) The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS ONE 6(5):e19595
Ryan ZC, Ketha H, McNulty MS et al (2013) Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci USA 110(15):6199–6204
Liu S, Quarles LD (2007) How fibroblast growth factor 23 works. J Am Soc Nephrol 18(6):1637–1647
Faul C, Amaral AP, Oskouei B et al (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121(11):4393–4408
Scialla JJ, Lau WL, Reilly MP et al (2013) Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 83(6):1159–1168
Haring R, Enserro D, Xanthakis V et al (2016) Plasma fibroblast growth factor 23: clinical correlates and association with cardiovascular disease and mortality in the Framingham heart study. J Am Heart Assoc 5:7
Adragao T, Herberth J, Monier-Faugere MC et al (2009) Low bone volume–a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol 4(2):450–455
Adragao T, Ferreira A, Frazao JM et al (2017) Higher mineralized bone volume is associated with a lower plain X-Ray vascular calcification score in hemodialysis patients. PLoS ONE 12(7):e0179868
Drechsler C, Evenepoel P, Vervloet MG et al (2015) High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant 30(2):288–293
Viaene L, Behets GJ, Claes K et al (2013) Sclerostin: another bone-related protein related to all-cause mortality in haemodialysis? Nephrol Dial Transplant 28(12):3024–3030
Claes KJ, Viaene L, Heye S, Meijers B, d’Haese P, Evenepoel P (2013) Sclerostin: another vascular calcification inhibitor? J Clin Endocrinol Metab 98(8):3221–3228
Goncalves FL, Elias RM, dos Reis LM et al (2014) Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol 15:190
Qureshi AR, Olauson H, Witasp A et al (2015) Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int 88(6):1356–1364
Lewiecki EM, Blicharski T, Goemaere S et al (2018) A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab 103(9):3183–3193
Acknowledgements
We thank CHULC, the institution where the study was conducted. We thank the Portuguese Society of Nephrology and the Portuguese Society of Transplantation/Astellas for awarding us a research grant. We would like to thank Sandra Silva for helping with administrative work and to Dr Isabel Mesquita for helping with sample storage. We also thank the surgeons Ana Pena and Sofia Carrelha and all the operating room staff for being available to help us with the bone biopsies performed in the operating room.
Funding
A grant from the Portuguese Society of Nephrology. A grant from the Portuguese Society of Transplantation/Astellas.
Author information
Authors and Affiliations
Contributions
ACF and AF concept and design the study; ACF, PC, IA, MM, DN, CS, FC, RS, BC, and GC collected data or perform bone biopsies or performed laboratory evaluations; ACF, MM, RS, BC, GC, FN, AF analyzed the data; ACF drafted the article; PC, IA, MM, DN, CS, FC, RS, BC, GC, FN, AF revised the paper; all authors approved the final version of the paper and agree with all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
Besides receiving a grant from the Portuguese Society of Nephrology and the Portuguese Society of Transplantation/Astellas, we do not have any conflict of interest relating with this manuscript. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Human and Animal Rights and Informed Consent
The institutions’ local ethics committees approved this study. Written consent was obtained from all participants prior to entering the protocol.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferreira, A.C., Cotovio, P., Aires, I. et al. The Role of Bone Volume, FGF23 and Sclerostin in Calcifications and Mortality; a Cohort Study in CKD Stage 5 Patients. Calcif Tissue Int 110, 215–224 (2022). https://doi.org/10.1007/s00223-021-00910-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00910-8