Abstract
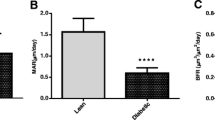
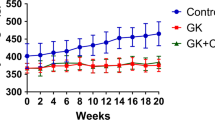
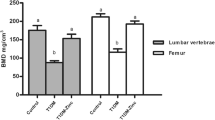
C-peptide therapy protects against diabetic micro- and macrovascular damages and neuropatic complications. However, to date, the role of C-peptide in preventing diabetes-related bone loss has not been investigated. Our aim was to evaluate if C-peptide infusion improves bone quality in diabetic rats. Twenty-three male Wistar rats were randomly divided into three groups: normal control group; sham diabetic control group; diabetic plus C-peptide group. Diabetes was induced by streptozotocin injection and C-peptide was delivered subcutaneously for 6 weeks. We performed micro-CT and histological testing to assess several trabecular microarchitectural parameters. At the end, diabetic plus C-peptide rats had a higher serum C-peptide (p = 0.02) and calcium (p = 0.04) levels and tibia weight (p = 0.02) than the diabetic control group. The diabetic plus C-peptide group showed a higher trabecular thickness and cross-sectional thickness than the diabetic control group (p = 0.01 and p = 0.03). Both the normal control and diabetic plus C-peptide groups had more Runx-2 and PLIN1 positive cells in comparison with the diabetic control group (p = 0.045 and p = 0.034). Diabetic rats receiving C-peptide had higher quality of trabecular bone than diabetic rats not receiving this treatment. If confirmed, C-peptide could have a role in improving bone quality in diabetes.




Similar content being viewed by others
References
Forsén L, Meyer HE, Midthjell K, Edna TH (1999) Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trøndelag Health Survey. Diabetologia 42(8):920–925. https://doi.org/10.1007/s001250051248
Léger J, Marinovic D, Alberti C, Dorgeret S, Chevenne D, Marchal CL, Tubiana-Rufi N, Sebag G, Czernichow P (2006) Lower bone mineral content in children with type 1 diabetes mellitus is linked to female sex, low insulin-like growth factor type I levels, and high insulin requirement. J Clin Endocrinol Metab 91(10):3947–3953. https://doi.org/10.1210/jc.2006-0711
Gunczler P, Lanes R, Paz-Martinez V, Martins R, Esaa S, Colmenares V, Weisinger JR (1998) Decreased lumbar spine bone mass and low bone turnover in children and adolescents with insulin dependent diabetes mellitus followed longitudinally. J Pediatric Endocrinol Metab JPEM 11(3):413–419. https://doi.org/10.1515/jpem.1998.11.3.413
Levin ME, Boisseau VC, Avioli LV (1976) Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med 294(5):241–245. https://doi.org/10.1056/NEJM197601292940502
Inzerillo AM, Epstein S (2004) Osteoporosis and diabetes mellitus. Rev Endocr Metab Disorders 5(3):261–268. https://doi.org/10.1023/B:REMD.0000032415.83124.20
Margeirsdottir HD, Stensaeth KH, Larsen JR, Brunborg C, Dahl-Jørgensen K (2010) Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care 33(9):2043–2048. https://doi.org/10.2337/dc10-0505
Costacou T, Lopes-Virella MF, Zgibor JC, Virella G, Otvos J, Walsh M, Orchard TJ (2005) Markers of endothelial dysfunction in the prediction of coronary artery disease in type 1 diabetes. The Pittsburgh Epidemiology of Diabetes Complications Study. J Diabetes Its Complicat 19(4):183–193. https://doi.org/10.1016/j.jdiacomp.2005.01.003
Campos Pastor MM, López-Ibarra PJ, Escobar-Jiménez F, Serrano Pardo MD, García-Cervigón AG (2000) Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: a prospective study. Osteoporosis Int 11(5):455–459. https://doi.org/10.1007/s001980070114
Nicodemus KK, Folsom AR, Iowa Women's Health Study (2001) Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 24(7):1192–1197. https://doi.org/10.2337/diacare.24.7.1192
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporosis Int 18(4):427–444. https://doi.org/10.1007/s00198-006-0253-4
Lu H, Kraut D, Gerstenfeld LC, Graves DT (2003) Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 144(1):346–352. https://doi.org/10.1210/en.2002-220072
Montalcini T, Romeo S, Ferro Y, Migliaccio V, Gazzaruso C, Pujia A (2013) Osteoporosis in chronic inflammatory disease: the role of malnutrition. Endocrine 43(1):59–64. https://doi.org/10.1007/s12020-012-9813-x
Suzuki K, Sugimoto C, Takizawa M, Ishizuka S, Kikuyama M, Togawa H, Taguchi Y, Nosaka K, Seino Y, Ishida H (2000) Correlations between bone mineral density and circulating bone metabolic markers in diabetic patients. Diabetes Res Clin Pract 48(3):185–191. https://doi.org/10.1016/s0168-8227(00)00119-4
Ekberg K, Brismar T, Johansson BL, Lindström P, Juntti-Berggren L, Norrby A, Berne C, Arnqvist HJ, Bolinder J, Wahren J (2007) C-Peptide replacement therapy and sensory nerve function in type 1 diabetic neuropathy. Diabetes Care 30(1):71–76. https://doi.org/10.2337/dc06-1274
Steiner DF, Cunningham D, Spigelman L, Aten B (1967) Insulin biosynthesis: evidence for a precursor. Science (New York, NY) 157(3789):697–700. https://doi.org/10.1126/science.157.3789.697
Hoogwerf BJ, Bantle JP, Gaenslen HE, Greenberg BZ, Senske BJ, Francis R, Goetz FC (1986) Infusion of synthetic human C-peptide does not affect plasma glucose, serum insulin, or plasma glucagon in healthy subjects. Metabolism 35(2):122–125. https://doi.org/10.1016/0026-0495(86)90111-3
Ekberg K, Brismar T, Johansson BL, Jonsson B, Lindström P, Wahren J (2003) Amelioration of sensory nerve dysfunction by C-Peptide in patients with type 1 diabetes. Diabetes 52(2):536–541. https://doi.org/10.2337/diabetes.52.2.536
Wahren J, Ekstrom U, Ekberg K (2011) C-peptide improves erectile function in type 1 diabetes. Diabetes 60:A285–A285
Hansen A, Johansson BL, Wahren J, von Bibra H (2002) C-peptide exerts beneficial effects on myocardial blood flow and function in patients with type 1 diabetes. Diabetes 51(10):3077–3082. https://doi.org/10.2337/diabetes.51.10.3077
Forst T, Kunt T, Pohlmann T, Goitom K, Engelbach M, Beyer J, Pfützner A (1998) Biological activity of C-peptide on the skin microcirculation in patients with insulin-dependent diabetes mellitus. J Clin Investig 101(10):2036–2041. https://doi.org/10.1172/JCI2147
Montalcini T, Gallotti P, Coppola A, Zambianchi V, Fodaro M, Galliera E, Marazzi MG, Romeo S, Giannini S, Corsi Romanelli MM, Pujia A, Gazzaruso C (2015) Association between low C-peptide and low lumbar bone mineral density in postmenopausal women without diabetes. Osteoporos Int 26(5):1639–1646. https://doi.org/10.1007/s00198-015-3040-2
Ferro Y, Russo C, Russo D, Gazzaruso C, Coppola A, Gallotti P, Zambianchi V, Fodaro M, Romeo S, Galliera E, Marazzi MG, Romanelli MMC, Giannini S, Pujia A, Montalcini T (2017) Association between low C-peptide and fragility fractures in postmenopausal women without diabetes. J Endocrinol Invest 40(10):1091–1098. https://doi.org/10.1007/s40618-017-0672-4
Russo C, Lazzaro V, Gazzaruso C, Maurotti S, Ferro Y, Pingitore P, Fumo F, Coppola A, Gallotti P, Zambianchi V, Fodaro M, Galliera E, Marazzi MG, Corsi Romanelli MM, Giannini S, Romeo S, Pujia A, Montalcini T (2017) Proinsulin C-peptide modulates the expression of ERK1/2, type I collagen and RANKL in human osteoblast-like cells (Saos-2). Mol Cell Endocrinol 442:134–141. https://doi.org/10.1016/j.mce.2016.12.012
Horcajada-Molteni MN, Chanteranne B, Lebecque P, Davicco MJ, Coxam V, Young A, Barlet JP (2001) Amylin and bone metabolism in streptozotocin-induced diabetic rats. J Bone Miner Res 16(5):958–965. https://doi.org/10.1359/jbmr.2001.16.5.958
Robey PG (1996) Vertebrate mineralized matrix proteins: structure and function. Connect Tissue Res 35(1–4):131–136. https://doi.org/10.3109/03008209609029183
Ingram RT, Clarke BL, Fisher LW, Fitzpatrick LA (1993) Distribution of noncollagenous proteins in the matrix of adult human bone: evidence of anatomic and functional heterogeneity. J Bone Miner Res 8(9):1019–1029. https://doi.org/10.1002/jbmr.5650080902
Anderson HC (2003) Matrix vesicles and calcification. Curr Rheumatol Rep 5(3):222–226. https://doi.org/10.1007/s11926-003-0071-z
Chen X, Goodman JM (2017) The collaborative work of droplet assembly. Biochim Biophys Acta Mol Cell Biol Lipids 1862(10):1205–1211. https://doi.org/10.1016/j.bbalip.2017.07.003
Samnegård B, Jacobson SH, Johansson BL, Ekberg K, Isaksson B, Wahren J, Sjöquist M (2004) C-peptide and captopril are equally effective in lowering glomerular hyperfiltration in diabetic rats. Nephrol Dialysis Transpl 19(6):1385–1391. https://doi.org/10.1093/ndt/gfh163
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25(7):1468–1486. https://doi.org/10.1002/jbmr.141
Ito M, Nakamura T, Matsumoto T, Tsurusaki K, Hayashi K (1998) Analysis of trabecular microarchitecture of human iliac bone using microcomputed tomography in patients with hip arthrosis with or without vertebral fracture. Bone 23(2):163–169. https://doi.org/10.1016/s8756-3282(98)00083-0
DalleCarbonare L, Valenti MT, Bertoldo F, Zanatta M, Zenari S, Realdi G, LoCascio V, Giannini S (2005) Bone microarchitecture evaluated by histomorphometry. Micron (Oxford, England: 1993) 36(7–8):609–616. https://doi.org/10.1016/j.micron.2005.07.007
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):2–17. https://doi.org/10.1002/jbmr.1805
Valenti MT, Giannini S, Donatelli L, Zanatta M, Bertoldo F, Sella S, Vilei MT, Ossi E, Realdi G, Lo Cascio V, Dalle Carbonare L (2010) The effect of risedronate on osteogenic lineage is mediated by cyclooxygenase-2 gene upregulation. Arthritis Res Therapy 12(4):R163. https://doi.org/10.1186/ar3122
Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C (1991) Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266(17):11341–11346
Gurel Pekozer G, Ramazanoglu M, Schlegel KA, Kok FN, Torun Kose G (2018) Role of STRO-1 sorting of porcine dental germ stem cells in dental stem cell-mediated bone tissue engineering. Artificial Cells Nanomed Biotechnol 46(3):607–618. https://doi.org/10.1080/21691401.2017.1332637
Müller R, van Lenthe H (2004) Microarchitectural aspects of quality and competence of bone. Adv Osteoporotic Fract Manag 3:2–12
Chen P, Jerome CP, Burr DB, Turner CH, Ma YL, Rana A, Sato M (2007) Interrelationships between bone microarchitecture and strength in ovariectomized monkeys treated with teriparatide. J Bone Miner Res 22(6):841–848. https://doi.org/10.1359/jbmr.070310
Doube M, Klosowski MM, Wiktorowicz-Conroy AM, Hutchinson JR, Shefelbine SJ (2011) Trabecular bone scales allometrically in mammals and birds. Proc Biol Sci 278(1721):3067–3073. https://doi.org/10.1098/rspb.2011.0069
Johansson BL, Sjöberg S, Wahren J (1992) The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia 35(2):121–128. https://doi.org/10.1007/BF00402543
Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J (2000) Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with Type 1 diabetes mellitus. Diabetic Med 17(3):181–189. https://doi.org/10.1046/j.1464-5491.2000.00274.x
Johansson BL, Linde B, Wahren J (1992) Effects of C-peptide on blood flow, capillary diffusion capacity and glucose utilization in the exercising forearm of type 1 (insulin-dependent) diabetic patients. Diabetologia 35(12):1151–1158. https://doi.org/10.1007/BF00401369
Gao X, Ma W, Dong H, Yong Z, Su R (2014) Establishing a rapid animal model of osteoporosis with ovariectomy plus low calcium diet in rats. Int J Clin Exp Pathol 7(8):5123–5128
Enríquez-Pérez IA, Galindo-Ordoñez KE, Pantoja-Ortíz CE, Martínez-Martínez A, Acosta-González RI, Muñoz-Islas E, Jiménez-Andrade JM (2017) Streptozocin-induced type-1 diabetes mellitus results in decreased density of CGRP sensory and TH sympathetic nerve fibers that are positively correlated with bone loss at the mouse femoral neck. Neurosci Lett 655:28–34. https://doi.org/10.1016/j.neulet.2017.06.042
Ferretti M, Cavani F, Roli L, Checchi M, Magarò MS, Bertacchini J, Palumbo C (2019) Interaction among calcium diet content, PTH (1–34) treatment and balance of bone homeostasis in rat model: the trabecular bone as keystone. Int J Mol Sci 20(3):753. https://doi.org/10.3390/ijms20030753
Nyman JS (2013) Effect of diabetes on the fracture resistance of bone. Clin Rev Bone Miner Metab 11(1):38–48
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, New MI, Zaidi M, Cinti S, Grano M (2015) The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA 112(39):12157–12162. https://doi.org/10.1073/pnas.1516622112
Bortolin RH, Freire Neto FP, Arcaro CA, Bezerra JF, da Silva FS, Ururahy MA, Souza KS, Lima VM, Luchessi AD, Lima FP, Lia Fook MV, da Silva BJ, Almeida MD, Abreu BJ, de Rezende LA, de Rezende AA (2017) Anabolic effect of insulin therapy on the bone: osteoprotegerin and osteocalcin up-regulation in streptozotocin-induced diabetic rats. Basic Clin Pharmacol Toxicol 120(3):227–234. https://doi.org/10.1111/bcpt.12672
Milovanovic P, Stojanovic M, Antonijevic D, Cirovic A, Radenkovic M, Djuric M (2018) "Dangerous duo": Chronic nicotine exposure intensifies diabetes mellitus-related deterioration in bone microstructure—an experimental study in rats. Life Sci 212:102–108. https://doi.org/10.1016/j.lfs.2018.09.044
Hua Y, Bi R, Zhang Y, Xu L, Guo J, Li Y (2018) Different bone sites-specific response to diabetes rat models: bone density, histology and microarchitecture. PLoS ONE 13(10):e0205503. https://doi.org/10.1371/journal.pone.0205503
Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34(1):195–202. https://doi.org/10.1016/j.bone.2003.10.001
Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Osteoporotic Fractures Research Group (2003) BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18(11):1947–1954. https://doi.org/10.1359/jbmr.2003.18.11.1947
Yeni YN, Brown CU, Wang Z, Norman TL (1997) The influence of bone morphology on fracture toughness of the human femur and tibia. Bone 21(5):453–459. https://doi.org/10.1016/s8756-3282(97)00173-7
Martin RB (1984) Porosity and specific surface of bone. Crit Rev Biomed Eng 10(3):179–222
Sornay-Rendu E, Boutroy S, Duboeuf F, Chapurlat RD (2017) Bone microarchitecture assessed by HR-pQCT as predictor of fracture risk in postmenopausal women: The OFELY Study. J Bone Miner Res 32(6):1243–1251. https://doi.org/10.1002/jbmr.3105
Shanbhogue VV, Hansen S, Frost M, Jørgensen NR, Hermann AP, Henriksen JE, Brixen K (2015) Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in adult patients with type 1 diabetes mellitus. J Bone Miner Res 30(12):2188–2199. https://doi.org/10.1002/jbmr.2573
Shanbhogue VV, Hansen S, Frost M, Jørgensen NR, Hermann AP, Henriksen JE, Brixen K (2016) Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol 174(2):115–124. https://doi.org/10.1530/EJE-15-0860
Golub EE (2009) Role of matrix vesicles in biomineralization. Biochem Biophys Acta 1790(12):1592–1598. https://doi.org/10.1016/j.bbagen.2009.09.006
Yamada Y, Ando F, Shimokata H (2006) Association of polymorphisms in forkhead box C2 and perilipin genes with bone mineral density in community-dwelling Japanese individuals. Int J Mol Med 18(1):119–127
Thomas MC, MacIsaac RJ, Tsalamandris C, Power D, Jerums G (2003) Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care 26(4):1164–1169. https://doi.org/10.2337/diacare.26.4.1164
Laudisio A, Marzetti E, Pagano F, Bernabei R, Zuccalà G (2009) Haemoglobin levels are associated with bone mineral density in the elderly: a population-based study. Clin Rheumatol 28(2):145–151. https://doi.org/10.1007/s10067-008-0998-6
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89(5):755–764. https://doi.org/10.1016/s0092-8674(00)80258-5
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425(6960):841–846. https://doi.org/10.1038/nature02040
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425(6960):836–841. https://doi.org/10.1038/nature02041
Anastasilakis AD, Goulis DG, Polyzos SA, Gerou S, Pavlidou V, Koukoulis G, Avramidis A (2008) Acute changes in serum osteoprotegerin and receptor activator for nuclear factor-kB ligand levels in women with established osteoporosis treated with teriparatide. Eur J Endocrinol 158(3):411–416
Shiraki M, Fukunaga M, Kushida K, Kishimoto H, Taketani Y, Minaguchi H, Inoue T, Morita R, Morii H, Yamamoto K, Ohashi Y, Orimo H (2003) A double-blind dose-ranging study of risedronate in Japanese patients with osteoporosis (a study by the Risedronate Late Phase II Research Group). Osteoporosis Int 14(3):225–234. https://doi.org/10.1007/s00198-002-1369-9
Acknowledgement
We are grateful to Dr John Wahren, from Karolinska Institutet, Stockholm, Sweden, for providing Rat C-Peptide.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SM, CR, MG, MS, FB, EM contributed to the experimental work. SM, CR and VM are guarantor of the integrity of the data; VM and SN performed microCT assessment; RP was responsible for the glucose monitoring and insulin injection planning; MTV, MD, SG and LDC performed the histological assessment; VMM, MR and DB performed all the laboratory measurements; SR, VM, AP and TM designed the study and prepared the first draft of the paper. TM and CG were responsible for statistical analysis of the data. All authors revised the paper critically for intellectual content and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
All authors declared there were no conflict of interests involved.
Ethical Approval
The experiments were carried out following the European guidelines (2010/63/EU) regarding procedures with animals used, in accordance with the approval of the Ethics Committee for Experimental Animals Welfare of the University Magna Grecia, Catanzaro (Auth. 10/01/2018) and Italian Ministry of Health (Auth. No 353/2018-protocol ADEAB.16, Auth. 9/05/2018).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maurotti, S., Russo, C., Musolino, V. et al. Effects of C-Peptide Replacement Therapy on Bone Microarchitecture Parameters in Streptozotocin-Diabetic Rats. Calcif Tissue Int 107, 266–280 (2020). https://doi.org/10.1007/s00223-020-00716-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00716-0