Abstract
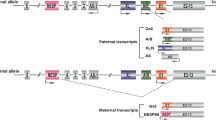
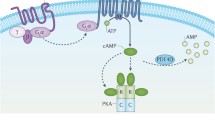
GNAS is one of the most complex gene loci in the human genome and encodes multiple gene products including Gsα, XLαs, NESP55, A/B, and AS transcripts. XLαs, the extra-large G protein ɑ-subunit, is paternally expressed. XLɑs and Gsɑ share the common 2–13 exons with different promoters and first exons. Therefore, XLɑs contains most of the functional domains of Gsα including receptor and effector binding sites. In vitro studies suggest a “Gsɑ”-like function of XLɑs regarding the stimulation of cAMP generation in response to receptor activation with different cellular actions. However, it is unclear whether XLαs has an important physiological function in humans. Pseudopseudohypoparathyroidism (PPHP) and progressive osseous heteroplasia (POH) are caused by paternally inherited mutations of GNAS. Maternal uniparental disomy of chromosome 20 [UPD(20)mat] lacks paternal chromosome 20. Therefore, the phenotypes of these diseases may be secondary to the abnormal functions of XLɑs, at least partly. From the phenotypes of human diseases like PPHP, POH, and UPD(20)mat, as well as some animal models with deficient XLɑs functions, it could be seen that XLɑs is involved in the growth and development of the mammalian fetus, plays a different role in glucose, lipid, and energy metabolism when compared with Gsɑ, and could prevent heterotopic ossification in humans and mice. More in vivo and in vitro studies, especially the development of conditional XLɑs knockout mice, are needed to clarify the physiopathologic roles and related signal pathways of XLɑs.

Similar content being viewed by others
References
Lemos MC, Thakker RV (2015) GNAS mutations in Pseudohypoparathyroidism type 1a and related disorders. Hum Mutat 36:11–19
Mantovani G, Bastepe M, Monk D, de Sanctis L, Thiele S, Usardi A, Ahmed SF, Bufo R, Choplin T, De Filippo G, Devernois G, Eggermann T, Elli FM, Freson K, Garcia Ramirez A, Germain-Lee EL, Groussin L, Hamdy N, Hanna P, Hiort O, Juppner H, Kamenicky P, Knight N, Kottler ML, Le Norcy E, Lecumberri B, Levine MA, Makitie O, Martin R, Martos-Moreno GA, Minagawa M, Murray P, Pereda A, Pignolo R, Rejnmark L, Rodado R, Rothenbuhler A, Saraff V, Shoemaker AH, Shore EM, Silve C, Turan S, Woods P, Zillikens MC, Perez de Nanclares G, Linglart A (2018) Diagnosis and management of pseudohypoparathyroidism and related disorders: first international consensus statement. Nat Rev Endocrinol 14:476–500
Levy-Shraga Y, Barel O, Javasky E, Barzilai A, Greenberger S (2019) Neonatal osteoma cutis due to a mutation in GNAS. Pediatr Dermatol 36:732–734
Turan S, Bastepe M (2015) GNAS Spectrum of Disorders. Curr Osteoporos Rep 13:146–158
Liu J, Erlichman B, Weinstein LS (2003) The stimulatory G protein alpha-subunit Gs alpha is imprinted in human thyroid glands: implications for thyroid function in pseudohypoparathyroidism types 1A and 1B. J Clin Endocrinol Metab 88:4336–4341
Lecumberri B, Fernandez-Rebollo E, Sentchordi L, Saavedra P, Bernal-Chico A, Pallardo LF, Bustos JM, Castano L, de Santiago M, Hiort O, Perez de Nanclares G, Bastepe M (2010) Coexistence of two different pseudohypoparathyroidism subtypes (Ia and Ib) in the same kindred with independent Gs{alpha} coding mutations and GNAS imprinting defects. J Med Genet 47:276–280
Crawford JA, Mutchler KJ, Sullivan BE, Lanigan TM, Clark MS, Russo AF (1993) Neural expression of a novel alternatively spliced and polyadenylated Gs alpha transcript. J Biol Chem 268:9879–9885
Pasolli HA, Klemke M, Kehlenbach RH, Wang Y, Huttner WB (2000) Characterization of the extra-large G protein alpha-subunit XLalphas. I. Tissue distribution and subcellular localization. J Biol Chem 275:33622–33632
Mantovani G, Spada A, Elli FM (2016) Pseudohypoparathyroidism and Gsalpha-cAMP-linked disorders: current view and open issues. Nat Rev Endocrinol 12:347–356
Liu Z, Turan S, Wehbi VL, Vilardaga JP, Bastepe M (2011) Extra-long Galphas variant XLalphas protein escapes activation-induced subcellular redistribution and is able to provide sustained signaling. J Biol Chem 286:38558–38569
Puzhko S, Goodyer CG, Kerachian MA, Canaff L, Misra M, Juppner H, Bastepe M, Hendy GN (2011) Parathyroid hormone signaling via Galphas is selectively inhibited by an NH(2)-terminally truncated Galphas: implications for pseudohypoparathyroidism. J Bone Miner Res 26:2473–2485
Klemke M, Pasolli HA, Kehlenbach RH, Offermanns S, Schultz G, Huttner WB (2000) Characterization of the extra-large G protein alpha-subunit XLalphas. II. Signal transduction properties. J Biol Chem 275:33633–33640
Linglart A, Mahon MJ, Kerachian MA, Berlach DM, Hendy GN, Juppner H, Bastepe M (2006) Coding GNAS mutations leading to hormone resistance impair in vitro agonist- and cholera toxin-induced adenosine cyclic 3',5'-monophosphate formation mediated by human XLalphas. Endocrinology 147:2253–2262
Bastepe M, Gunes Y, Perez-Villamil B, Hunzelman J, Weinstein LS, Juppner H (2002) Receptor-mediated adenylyl cyclase activation through XLalpha(s), the extra-large variant of the stimulatory G protein alpha-subunit. Mol Endocrinol 16:1912–1919
Aydin C, Aytan N, Mahon MJ, Tawfeek HA, Kowall NW, Dedeoglu A, Bastepe M (2009) Extralarge XL(alpha)s (XXL(alpha)s), a variant of stimulatory G protein alpha-subunit (Gs(alpha)), is a distinct, membrane-anchored GNAS product that can mimic Gs(alpha). Endocrinology 150:3567–3575
Chen M, Gavrilova O, Liu J, Xie T, Deng C, Nguyen AT, Nackers LM, Lorenzo J, Shen L, Weinstein LS (2005) Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci USA 102:7386–7391
Plagge A, Gordon E, Dean W, Boiani R, Cinti S, Peters J, Kelsey G (2004) The imprinted signaling protein XL alpha s is required for postnatal adaptation to feeding. Nat Genet 36:818–826
Xie T, Plagge A, Gavrilova O, Pack S, Jou W, Lai EW, Frontera M, Kelsey G, Weinstein LS (2006) The alternative stimulatory G protein alpha-subunit XLalphas is a critical regulator of energy and glucose metabolism and sympathetic nerve activity in adult mice. J Biol Chem 281:18989–18999
Kehlenbach RH, Matthey J, Huttner WB (1994) XL alpha s is a new type of G protein. Nature 372:804–809
Abramowitz J, Grenet D, Birnbaumer M, Torres HN, Birnbaumer L (2004) XLalphas, the extra-long form of the alpha-subunit of the Gs G protein, is significantly longer than suspected, and so is its companion Alex. Proc Natl Acad Sci USA 101:8366–8371
Mariot V, Wu JY, Aydin C, Mantovani G, Mahon MJ, Linglart A, Bastepe M (2011) Potent constitutive cyclic AMP-generating activity of XLalphas implicates this imprinted GNAS product in the pathogenesis of McCune-Albright syndrome and fibrous dysplasia of bone. Bone 48:312–320
Kaya AI, Ugur O, Oner SS, Bastepe M, Onaran HO (2009) Coupling of beta2-adrenoceptors to XLalphas and Galphas: a new insight into ligand-induced G protein activation. J Pharmacol Exp Ther 329:350–359
Pignolo RJ, Xu M, Russell E, Richardson A, Kaplan J, Billings PC, Kaplan FS, Shore EM (2011) Heterozygous inactivation of Gnas in adipose-derived mesenchymal progenitor cells enhances osteoblast differentiation and promotes heterotopic ossification. J Bone Miner Res 26:2647–2655
Weinstein LS, Yu S, Warner DR, Liu J (2001) Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev 22:675–705
Vallar L (1990) GTPase-inhibiting mutations activate the alpha-chain of Gs in human tumours. Biochem Soc Symp 56:165–170
Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L (1989) GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340:692–696
Idowu BD, Al-Adnani M, O'Donnell P, Yu L, Odell E, Diss T, Gale RE, Flanagan AM (2007) A sensitive mutation-specific screening technique for GNAS1 mutations in cases of fibrous dysplasia: the first report of a codon 227 mutation in bone. Histopathology 50:691–704
Eaton SA, Williamson CM, Ball ST, Beechey CV, Moir L, Edwards J, Teboul L, Maconochie M, Peters J (2012) New mutations at the imprinted Gnas cluster show gene dosage effects of Gsalpha in postnatal growth and implicate XLalphas in bone and fat metabolism but not in suckling. Mol Cell Biol 32:1017–1029
Hanna P, Grybek V, Perez de Nanclares G, Tran LC, de Sanctis L, Elli F, Errea J, Francou B, Kamenicky P, Linglart L, Pereda A, Rothenbuhler A, Tessaris D, Thiele S, Usardi A, Shoemaker AH, Kottler ML, Juppner H, Mantovani G, Linglart A (2018) Genetic and epigenetic defects at the GNAS locus lead to distinct patterns of skeletal growth but similar early-onset obesity. J Bone Miner Res 33:1480–1488
He Q, Zhu Y, Corbin BA, Plagge A, Bastepe M (2015) The G protein alpha subunit variant XLalphas promotes inositol 1,4,5-trisphosphate signaling and mediates the renal actions of parathyroid hormone in vivo. Sci Signal 8:84
Zheng H, Radeva G, McCann JA, Hendy GN, Goodyer CG (2001) Galphas transcripts are biallelically expressed in the human kidney cortex: implications for pseudohypoparathyroidism type 1b. J Clin Endocrinol Metab 86:4627–4629
Li T, Vu TH, Zeng ZL, Nguyen BT, Hayward BE, Bonthron DT, Hu JF, Hoffman AR (2000) Tissue-specific expression of antisense and sense transcripts at the imprinted Gnas locus. Genomics 69:295–304
Pasolli HA, Huttner WB (2001) Expression of the extra-large G protein alpha-subunit XLalphas in neuroepithelial cells and young neurons during development of the rat nervous system. Neurosci Lett 301:119–122
Liu Z, Segawa H, Aydin C, Reyes M, Erben RG, Weinstein LS, Chen M, Marshansky V, Frohlich LF, Bastepe M (2011) Transgenic overexpression of the extra-large Gsalpha variant XLalphas enhances Gsalpha-mediated responses in the mouse renal proximal tubule in vivo. Endocrinology 152:1222–1233
Takatani R, Minagawa M, Molinaro A, Reyes M, Kinoshita K, Takatani T, Kazukawa I, Nagatsuma M, Kashimada K, Sato K, Matsushita K, Nomura F, Shimojo N, Juppner H (2015) Similar frequency of paternal uniparental disomy involving chromosome 20q (patUPD20q) in Japanese and Caucasian patients affected by sporadic pseudohypoparathyroidism type Ib (sporPHP1B). Bone 79:15–20
Bastepe M, Lane AH, Juppner H (2001) Paternal uniparental isodisomy of chromosome 20q–and the resulting changes in GNAS1 methylation–as a plausible cause of pseudohypoparathyroidism. Am J Hum Genet 68:1283–1289
Mulchandani S, Bhoj EJ, Luo M, Powell-Hamilton N, Jenny K, Gripp KW, Elbracht M, Eggermann T, Turner CL, Temple IK, Mackay DJ, Dubbs H, Stevenson DA, Slattery L, Zackai EH, Spinner NB, Krantz ID, Conlin LK (2016) Maternal uniparental disomy of chromosome 20: a novel imprinting disorder of growth failure. Genet Med 18:309–315
Skinner JA, Cattanach BM, Peters J (2002) The imprinted oedematous-small mutation on mouse chromosome 2 identifies new roles for Gnas and Gnasxl in development. Genomics 80:373–375
Chen M, Haluzik M, Wolf NJ, Lorenzo J, Dietz KR, Reitman ML, Weinstein LS (2004) Increased insulin sensitivity in paternal Gnas knockout mice is associated with increased lipid clearance. Endocrinology 145:4094–4102
Germain-Lee EL, Schwindinger W, Crane JL, Zewdu R, Zweifel LS, Wand G, Huso DL, Saji M, Ringel MD, Levine MA (2005) A mouse model of albright hereditary osteodystrophy generated by targeted disruption of exon 1 of the Gnas gene. Endocrinology 146:4697–4709
Kelly ML, Moir L, Jones L, Whitehill E, Anstee QM, Goldin RD, Hough A, Cheeseman M, Jansson JO, Peters J, Cox RD (2009) A missense mutation in the non-neural G-protein alpha-subunit isoforms modulates susceptibility to obesity. Int J Obes (London) 33:507–518
Han SR, Lee YA, Shin CH, Yang SW, Lim BC, Cho TJ, Ko JM (2019) Clinical and molecular characteristics of GNAS inactivation disorders observed in 18 Korean patients. Exp Clin Endocrinol Diabetes. https://doi.org/10.1055/a-1001-3575
Richard N, Molin A, Coudray N, Rault-Guillaume P, Juppner H, Kottler ML (2013) Paternal GNAS mutations lead to severe intrauterine growth retardation (IUGR) and provide evidence for a role of XLalphas in fetal development. J Clin Endocrinol Metab 98:E1549–1556
Adegbite NS, Xu M, Kaplan FS, Shore EM, Pignolo RJ (2008) Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am J Med Genet A 146A:1788–1796
Kaplan FS, Craver R, MacEwen GD, Gannon FH, Finkel G, Hahn G, Tabas J, Gardner RJ, Zasloff MA (1994) Progressive osseous heteroplasia: a distinct developmental disorder of heterotopic ossification. Two new case reports and follow-up of three previously reported cases. J Bone Joint Surg Am 76:425–436
Lebrun M, Richard N, Abeguile G, David A, Coeslier Dieux A, Journel H, Lacombe D, Pinto G, Odent S, Salles JP, Taieb A, Gandon-Laloum S, Kottler ML (2010) Progressive osseous heteroplasia: a model for the imprinting effects of GNAS inactivating mutations in humans. J Clin Endocrinol Metab 95:3028–3038
Colson C, Decamp M, Gruchy N, Coudray N, Ballandonne C, Bracquemart C, Molin A, Mittre H, Takatani R, Juppner H, Kottler ML, Richard N (2019) High frequency of paternal iso or heterodisomy at chromosome 20 associated with sporadic pseudohypoparathyroidism 1B. Bone 123:145–152
Bastepe M, Altug-Teber O, Agarwal C, Oberfield SE, Bonin M, Juppner H (2011) Paternal uniparental isodisomy of the entire chromosome 20 as a molecular cause of pseudohypoparathyroidism type Ib (PHP-Ib). Bone 48:659–662
Kawashima S, Nakamura A, Inoue T, Matsubara K, Horikawa R, Wakui K, Takano K, Fukushima Y, Tatematsu T, Mizuno S, Tsubaki J, Kure S, Matsubara Y, Ogata T, Fukami M, Kagami M (2018) Maternal uniparental disomy for chromosome 20: physical and endocrinological characteristics of five patients. J Clin Endocrinol Metab 103:2083–2088
Chudoba I, Franke Y, Senger G, Sauerbrei G, Demuth S, Beensen V, Neumann A, Hansmann I, Claussen U (1999) Maternal UPD 20 in a hyperactive child with severe growth retardation. Eur J Hum Genet 7:533–540
Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL (2007) Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Galpha(s) in the development of human obesity. J Clin Endocrinol Metab 92:1073–1079
Funding
This work was supported by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYGD18022 to Haoming Tian).
Author information
Authors and Affiliations
Contributions
YW and XC wrote the paper. HT revised the paper.
Corresponding author
Ethics declarations
Conflict of interest
Yan Wang, Xiang Chen, and Haoming Tian declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Tian, H. & Chen, X. The Distinct Role of the Extra-Large G Protein ɑ-Subunit XLɑs. Calcif Tissue Int 107, 212–219 (2020). https://doi.org/10.1007/s00223-020-00714-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00714-2