Abstract
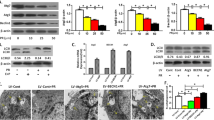
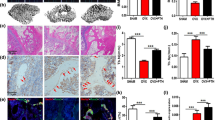
Autophagy is an evolutionarily conserved dynamic process and present in variety of cells at basal levels to maintain homeostasis and to promote cell survival in response to stresses. The early bone loss with excessive glucocorticoids (GCs) was reported to be related with the extension of the life span of osteoclasts. However, the connection between GCs induced bone loss and osteoclast autophagy remains to be elucidated. Autophagy was detected in a Dexamethasone (Dex) induced osteoporotic mice model and primary osteoclast cultures by autophagosome detection kit, and autophagy-related proteins were assayed by Western blotting and Immunostaining. The bone morphology was examined by micro-CT and TRAP staining. The trabecular bone micro-architecture was deteriorated, and the osteoclast number and spread area were increased in the Dex-treated mice compared with the control group (P < 0.01). Meanwhile, autophagy in pre-osteoclasts was increased in mice under Dex administration evidenced by the increased number of autophagosome and up-regulation of autophagy-related protein levels. Further, the enhanced autophagy under Dex treatment was verified in primary cultured osteoclasts, as shown by the increased levels of Beclin 1 and LC3-II/LC3-I and the autophagy complex formation members including Atg1, Atg13, and Atg7. However, the expressions of PI3K, p-Akt and p-mTOR in primary cultured osteoclasts were inhibited under Dex induced autophagy. Using the selective PTEN inhibitor SF1670 to activate the PI3K/Akt/mTOR pathway reversed this osteoclast autophagy under Dex treatment. Our study suggests that osteoclast autophagy was enhanced in glucocorticoids induced bone loss, and the PI3K/Akt/mTOR signaling pathway mediated the increased autophagy in primary cultured osteoclasts under glucocorticoids treatment.






Similar content being viewed by others
References
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C, Govoni M, Houssiau F, Jayne D, Kouloumas M, Kuhn A, Larsen JL, Lerstrøm K, Moroni G, Mosca M, Schneider M, Smolen JS, Svenungsson E, Tesar V, Tincani A, Troldborg A, van Vollenhoven R, Wenzel J, Bertsias G, Boumpas DT (2019) 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 78:736–745. https://doi.org/10.1136/annrheumdis-2019-215089
Adami G, Saag KG (2019) Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int 30:1145–1156. https://doi.org/10.1007/s00198-019-04906-x
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Robinson AB, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T (2017) 2017 American college of rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol 69:1521–1537. https://doi.org/10.1002/art.40137
Hardy RS, Zhou H, Seibel MJ, Cooper MS (2018) Glucocorticoids and bone: consequences of endogenous and exogenous excess and replacement therapy. Endocr Rev 39:519–548. https://doi.org/10.1210/er.2018-00097
Cappariello A, Maurizi A, Veeriah V, Teti A (2014) The great beauty of the osteoclast. Arch Biochem Biophys 558:70–78. https://doi.org/10.1016/j.abb.2014.06.017
Jia D, O'Brien CA, Stewart SA, Manolagas SC, Weinstein RS (2006) Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology 147:5592–5599. https://doi.org/10.1210/en.2006-0459
Den Uyl D, Bultink IE, Lems WF (2011) Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep 13:233–240. https://doi.org/10.1007/s11926-011-0173-y
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 102:274–282. https://doi.org/10.1172/JCI2799
Kondo N, Tokunaga K, Ito T, Arai K, Amizuka N, Minqi L, Kitahara H, Ito M, Naito M, Shu-Ying J, Oda K, Murai T, Takano R, Ogose A, Endo N (2006) High dose glucocorticoid hampers bone formation and resorption after bone marrow ablation in rat. Microsc Res Tech 69:839–846. https://doi.org/10.1002/jemt.20355
Kim HJ, Zhao H, Kitaura H, Bhattacharyya S, Brewer JA, Muglia LJ, Ross FP, Teitelbaum SL (2006) Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest 116:2152–2160. https://doi.org/10.1172/JCI28084
Zhang L, Guo YF, Liu YZ, Liu YJ, Xiong DH, Liu XG, Wang L, Yang TL, Lei SF, Guo Y, Yan H, Pei YF, Zhang F, Papasian CJ, Recker RR, Deng HW (2010) Pathway-based genome-wide association analysis identified the importance of regulation-of-autophagy pathway for ultradistal radius BMD. J Bone Miner Res 25:1572–1580. https://doi.org/10.1002/jbmr.36
Lin NY, Chen CW, Kagwiria R, Liang R, Beyer C, Distler A, Luther J, Engelke K, Schett G, Distler JH (2016) Inactivation of autophagy ameliorates glucocorticoid-induced and ovariectomy-induced bone loss. Ann Rheum Dis 75:1203–1210. https://doi.org/10.1136/annrheumdis-2015-207240
Cuomo F, Altucci L, Cobellis G (2019) Autophagy function and dysfunction: potential drugs as anti-cancer therapy. Cancers 11:E1465. https://doi.org/10.3390/cancers11101465
Jia J, Yao W, Guan M, Dai W, Shahnazari M, Kar R, Bonewald L, Jiang JX, Lane NE (2011) Glucocorticoid dose determines osteocyte cell fate. FASEB J 25:3366–3376. https://doi.org/10.1096/fj.11-182519
Arai A, Kim S, Goldshteyn V, Kim T, Park NH, Wang CY, Kim RH (2019) Beclin1 modulates bone homeostasis by regulating osteoclast and chondrocyte differentiation. J Bone Miner Res 34:1753–1766. https://doi.org/10.1002/jbmr.3756
Pierrefite-Carle V, Santucci-Darmanin S, Breuil V, Camuzard O, Carle GF (2015) Autophagy in bone: self-eating to stay in balance. Ageing Res Rev 24:206–217. https://doi.org/10.1016/j.arr.2015.08.004
DeSelm CJ, Miller BC, Zou W, Beatty WL, van Meel E, Takahata Y, Klumperman J, Tooze SA, Teitelbaum SL, Virgin HW (2011) Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell 21:966–974. https://doi.org/10.1016/j.devcel.2011.08.016
Chung YH, Jang Y, Choi B, Song DH, Lee EJ, Kim SM, Song Y, Kang SW, Yoon SY, Chang EJ (2014) Beclin-1 is required for RANKL-induced osteoclast differentiation. J Cell Physiol 229:1963–1971. https://doi.org/10.1002/jcp.24646
Chung YH, Yoon SY, Choi B, Sohn DH, Yoon KH, Kim WJ, Kim DH, Chang EJ (2012) Microtubule-associated protein light chain 3 regulates Cdc42-dependent actin ring formation in osteoclast. Int J Biochem Cell Biol 44:989–997. https://doi.org/10.1016/j.biocel.2012.03.007
Nollet M, Santucci-Darmanin S, Breuil V, Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S, Cailleteau L, Battaglia S, Farlay D, Dacquin R, Barois N, Jurdic P, Boivin G, Heymann D, Lafont F, Lu SS, Dempster DW, Carle GF, Pierrefite-Carle V (2014) Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 10:1965–1977. https://doi.org/10.4161/auto.36182
Lin NY, Beyer C, Giessl A, Kireva T, Scholtysek C, Uderhardt S, Munoz LE, Dees C, Distler A, Wirtz S, Krönke G, Spencer B, Distler O, Schett G, Distler JH (2013) Autophagy regulates TNFα-mediated joint destruction in experimental arthritis. Ann Rheum Dis 72:761–768. https://doi.org/10.1136/annrheumdis-2012-201671
Shi J, Wang L, Zhang H, Jie Q, Li X, Shi Q, Huang Q, Gao B, Han Y, Guo K, Liu J, Yang L, Luo Z (2015) Glucocorticoids: Dose-related effects on osteoclast formation and function via reactive oxygen species and autophagy. Bone 79:222–232. https://doi.org/10.1016/j.bone.2015.06.014
Li Y, Sun R, Zou J, Ying Y, Luo Z (2019) Dual roles of the AMP-activated protein kinase pathway in angiogenesis. Cells 8:E752. https://doi.org/10.3390/cells8070752
Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26:2694–2701. https://doi.org/10.1016/j.cellsig.2014.08.019
Ma J, Du D, Liu J, Guo L, Li Y, Chen A, Ye T (2020) Hydrogen sulphide promotes osteoclastogenesis by inhibiting autophagy through the PI3K/AKT/mTOR pathway. J Drug Target 28:176–185. https://doi.org/10.1080/1061186X.2019.1624969
Gan ZY, Fitter S, Vandyke K, To LB, Zannettino AC, Martin SK (2015) The effect of the dual PI3K and mTOR inhibitor BEZ235 on tumour growth and osteolytic bone disease in multiple myeloma. Eur J Haematol 94:343–354. https://doi.org/10.1111/ejh.12436
Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schütz G, Tuckermann JP (2010) Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 11:517–531. https://doi.org/10.1016/j.cmet.2010.05.005
Le Nihouannen D, Barralet JE, Fong JE, Komarova SV (2010) Ascorbic acid accelerates osteoclast formation and death. Bone 46:1336–1343. https://doi.org/10.1016/j.bone.2009.11.021
Zhao W, Byrne MH, Boyce BF, Krane SM (1999) Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest 103:517–524. https://doi.org/10.1172/JCI5481
Kaur G, Ahn J, Hankenson KD, Ashley JW (2017) Stimulation of notch signaling in mouse osteoclast precursors. J Vis Exp 120:e55234. https://doi.org/10.3791/55234
Zhang Y, Xu S, Li K, Tan K, Liang K, Wang J, Shen J, Zou W, Hu L, Cai D, Ding C, Li M, Xiao G, Liu B, Liu A, Bai X (2017) mTORC1 Inhibits NF-κB/NFATc1 signaling and prevents osteoclast precursor differentiation, in vitro and in mice. J Bone Miner Res 32:1829–1840. https://doi.org/10.1002/jbmr.3172
Weinstein RS (2012) Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am 41:595–611. https://doi.org/10.1016/j.ecl.2012.04.004
Wang K, Niu J, Kim H, Kolattukudy PE (2011) Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress, and autophagy. J Mol Cell Biol 3:360–368. https://doi.org/10.1093/jmcb/mjr021
Sambandam Y, Townsend MT, Pierce JJ, Lipman CM, Haque A, Bateman TA, Reddy SV (2014) Microgravity control of autophagy modulates osteoclastogenesis. Bone 61:125–131. https://doi.org/10.1016/j.bone.2014.01.004
Zhao Y, Chen G, Zhang W, Xu N, Zhu JY, Jia J, Sun ZJ, Wang YN, Zhao YF (2012) Autophagy regulates hypoxia-induced osteoclastogenesis through the HIF-1α/BNIP3 signaling pathway. J Cell Physiol 227:639–648. https://doi.org/10.1002/jcp.22768
Vanderoost J, Søe K, Merrild DM, Delaissé JM, van Lenthe GH (2013) Glucocorticoid-induced changes in the geometry of osteoclast resorption cavities affect trabecular bone stiffness. Calcif Tissue Int 92:240–250. https://doi.org/10.1007/s00223-012-9674-6
Farahmand P, Marin F, Hawkins F, Möricke R, Ringe JD, Glüer CC, Papaioannou N, Minisola S, Martínez G, Nolla JM, Niedhart C, Guañabens N, Nuti R, Martín-Mola E, Thomasius F, Peña J, Graeff C, Kapetanos G, Petto H, Gentzel A, Reisinger A, Zysset PK (2013) Early changes in biochemical markers of bone formation during teriparatide therapy correlate with improvements in vertebral strength in men with glucocorticoid-induced osteoporosis. Osteoporos Int 24:2971–2981. https://doi.org/10.1007/s00198-013-2379-5
Adami G, Rahn EJ, Saag KG (2019) Glucocorticoid-induced osteoporosis: from clinical trials to clinical practice. Ther Adv Musculoskelet Dis 11:1759720X19876468. https://doi.org/10.1177/1759720X19876468
Levy JMM, Towers CG, Thorburn A (2017) Targeting autophagy in cancer. Nat Rev Cancer 17:528–542. https://doi.org/10.1038/nrc.2017.53
Sciarretta S, Maejima Y, Zablocki D, Sadoshima J (2018) The role of autophagy in the heart. Annu Rev Physiol 80:1–26. https://doi.org/10.1146/annurev-physiol-021317-121427
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Łos MJ (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49. https://doi.org/10.1016/j.pneurobio.2013.10.004
Luo D, Ren H, Li T, Lian K, Lin D (2016) Rapamycin reduces severity of senile osteoporosis by activating osteocyte autophagy. Osteoporos Int 27:1093–1101. https://doi.org/10.1007/s00198-015-3325-5
Homewood CA, Warhurst DC, Peters W, Baggaley VC (1972) Lysosomes, pH and the anti-malarial action of chloroquine. Nature 235:50–52. https://doi.org/10.1038/235050a0
Mok CC, Mak A, Ma KM (2005) Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus 14:106–112. https://doi.org/10.1191/0961203305lu2039oa
Lakshminarayanan S, Walsh S, Mohanraj M, Rothfield N (2001) Factors associated with low bone mineral density in female patients with systemic lupus erythematosus. J Rheumatol 28:102–108
Li W, Zhang S, Liu J, Liu Y, Liang Q (2019) Vitamin K2 stimulates MC3T3 E1 osteoblast differentiation and mineralization through autophagy induction. Mol Med Rep 19:3676–3684. https://doi.org/10.3892/mmr.2019.10040
Park HJ, Son HJ, Sul OJ, Suh JH, Choi HS (2018) 4-Phenylbutyric acid protects against lipopolysaccharide-induced bone loss by modulating autophagy in osteoclasts. Biochem Pharmacol 151:9–17. https://doi.org/10.1016/j.bcp.2018.02.019
Tsai CH, Hsu MH, Huang PH, Hsieh CT, Chiu YM, Shieh DC, Lee YJ, Tsay GJ, Wu YY (2018) A paeonol derivative, YPH-PA3 promotes the differentiation of monocyte/macrophage lineage precursor cells into osteoblasts and enhances their autophagy. Eur J Pharmacol 832:104–113. https://doi.org/10.1016/j.ejphar.2018.05.024
Cheng X, Zhu L, Zhang J, Yu J, Liu S, Lv F, Lin Y, Liu G, Peng B (2017) Anti-osteoclastogenesis of mineral trioxide aggregate through inhibition of the autophagic pathway. J Endod 43:766–773. https://doi.org/10.1016/j.joen.2016.12.013
Liu S, Zhu L, Zhang J, Yu J, Cheng X, Peng B (2016) Anti-osteoclastogenic activity of isoliquiritigenin via inhibition of NF-κB-dependent autophagic pathway. Biochem Pharmacol 106:82–93. https://doi.org/10.1016/j.bcp.2016.03.002
Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y (1998) A protein conjugation system essential for autophagy. Nature 395:395–398. https://doi.org/10.1038/26506
Li RF, Chen G, Ren JG, Zhang W, Wu ZX, Liu B, Zhao Y, Zhao YF (2014) The adaptor protein p62 is involved in RANKL-induced autophagy and osteoclastogenesis. J Histochem Cytochem 62:879–888. https://doi.org/10.1369/0022155414551367
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant No. 81201424), the Projects of Shanghai Municipal Health Bureau (Grant No. 20124332), Shanghai Clinical Medical Center (Grant No. 2017ZZ01023) and Shanghai Municipal Key Clinical Specialty.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LF, WW and XS. The first draft of the manuscript was written by LF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted and ethical approval was obtained from Ethics Committee and the Institutional Animal Care and Use Committee of Shanghai Ninth People’s Hospital.
Human and Animal Rights and Informed Consent
The Animal Care and Use Committee of Shanghai Ninth People’s Hospital approved the use of animals in this study. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, L., Wu, W., Sun, X. et al. Glucocorticoids Enhanced Osteoclast Autophagy Through the PI3K/Akt/mTOR Signaling Pathway. Calcif Tissue Int 107, 60–71 (2020). https://doi.org/10.1007/s00223-020-00687-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00687-2