Abstract
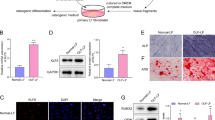
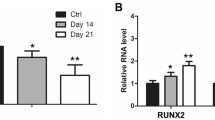
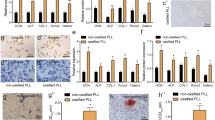
Ossification of the ligamentum flavum (OLF) is characterized by a process of ectopic bone formation in the ligamentum flavum. The definitive pathophysiology of OLF still remains unclear, but the epigenetic m6A modification plays an important role in OLF. In addition, no studies have reported the function of ALKBH5 in OLF development. In this study, we investigated the function of the m6A demethylation enzyme ALKBH5 in OLF. To evaluate the function of ALKBH5, OLF tissues and normal ligamentum flavum tissues were collected. In vitro methods, including HE, IHC and western blotting assays, were used to evaluate the association of ALKBH5 with OLF. In addition, we verified the effects of ALKBH5 on osteogenesis using alizarin red and ALP staining. MeRIP q-PCR was performed to investigate the methylation level of BMP2. Moreover, the mechanism of ALKBH5-mediated regulation of the ossification of the ligamentum flavum cells through the AKT signaling pathway was also verified. The present study showed that the expression of ALKBH5 increased in OLF tissues. The overexpression of ALKBH5 increased the expression of osteogenic genes and promoted the ossification of ligamentum flavum cells. Furthermore, BMP2 was significantly enriched in the ligamentum flavum cells of the anti-m6A group compared with those of the IgG group. The overexpression of ALKBH5 led to the activation of p-AKT, and BMP2 was regulated by ALKBH5 through the AKT signaling pathway. ALKBH5 promoted the osteogenesis of the ligamentum flavum cells through BMP2 demethylation and AKT activation. ALKBH5 was shown to be an important demethylation enzyme in OLF development.




Similar content being viewed by others
Change history
01 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00223-024-01206-3
References
Polgar F (1920) Uber interakuelle wirbelverkalkung. Fortschr Geb Rongenstr Nuklearmed Erganzungsband 40:292–298
Miyasaka K, Kaneda K, Sato S, Iwasaki Y, Abe S, Takei H, Tsuru M, Tashiro K, Abe H, Fujioka Y (1900) Myelopathy due to ossification or calcification of the ligamentum flavum: radiologic and histologic evaluations. AJNR Am J Neuroradiol 4:629–632
Tokala D, Lam K, Prince HG (2007) Ossification of the proximal thoracic ligamenta flava causing acute myelopathy in a Caucasian: case report and literature review. Spinal Cord 45:310–313
Guo JJ, Luk KD, Karppinen J, Yang H, Cheung KM (2010) Prevalence, distribution, and morphology of ossification of the ligamentum flavum: a population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine 35:51–56
Hai L, Lei-Sheng J, Li-Yang D (2007) Hormones and growth factors in the pathogenesis of spinal ligament ossification. Eur Spine J 16:1075–1084
Hanakita J, Suwa H, Ohta F, Nishi S, Sakaida H, Iihara K (1990) Neuroradiological examination of thoracic radiculo-myelopathy due to ossification of the ligamentum flavum. Neuroradiology 32:38
Kim HN, Min WK, Jeong JH, Kim SG, Kim JR, Kim SY, Choi JY, Park BC (2011) Combination of Runx2 and BMP2 increases conversion of human ligamentum flavum cells into osteoblastic cells. BMB Rep 44:446–451
Yayama T, Uchida K, Kobayashi S, Kokubo Y, Sato R (2007) Thoracic ossification of the human ligamentum flavum: histopathological and immunohistochemical findings around the ossified lesion. J Neurosurg Spine 7:184–193
Roundtree IA, Evans ME, Pan T, He C (2017) Dynamic RNA modifications in gene expression regulation. Cell 169:1187–1200
Zhao BS, Roundtree IA, He C (2017) Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 18:31–42
Fu Y, Dominissini D, Rechavi G, He C (2014) Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet 15:293–306
Desrosiers R, Friderici K, Rottman F (1974) Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA 71:3971–3975
Panneerdoss S, Eedunuri VK, Yadav P, Timilsina S, Rajamanickam S, Viswanadhapalli S, Abdelfattah N (2018) Cross-talk among writers, readers, and erasers of m(6)A regulates cancer growth and progression. Sci Adv 4:eaar8263
Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, Wong CC, Ng IO, Wong CM (2017) RNA N6-methyladenosine methyltransferase METTL3 promotes liver cancer progression through YTHDF2 dependent post-transcriptional silencing of SOCS2. Hepatology. https://doi.org/10.1002/hep.29683
Frye M, Harada BT (2018) RNA modifications modulate gene expression during development. Science 361:1346–1349
Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY, Yang D, Zheng ZC, Zhao Y (2019) ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J Physiol Biochem 75:379–389
Zhong ZM, Chen JT (2009) Phenotypic characterization of ligamentum flavum cells from patients with ossification of ligamentum flavum. Yonsei Med J 50:375–379
Kuang M-J, Zhang W-H, He W-W, Sun L, Ma J-X, Wang D, Ma X-l (2019) Naringin regulates bone metabolism in glucocorticoid-induced osteonecrosis of the femoral head via the Akt/Bad signal cascades. Chemico-biol Interact 304:97–105
Albrechtova J, Kubinova Z, Soukup A, Janacek J (2019) Image analysis: basic procedures for description of plant structures. Methods Mol Biol 1992:109–119
Georges R, Béatrice V, Fred D, Roland B, Sergio RR (2010) BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res 18:1842–1853
Kuang MJ, Xing F, Wang D, Sun L, Ma JX, Ma XL (2019) CircUSP45 inhibited osteogenesis in glucocorticoid-induced osteonecrosis of femoral head by sponging miR-127-5p through PTEN/AKT signal pathway: experimental studies. Biochem Biophys Res Commun 509:255–261
Bertrand J, Nitschke Y, Fuerst M, Hermann S, Schafers M, Sherwood J, Nalesso G, Ruether W, Rutsch F, Dell'Accio F, Pap T (2012) Decreased levels of nucleotide pyrophosphatase phosphodiesterase 1 are associated with cartilage calcification in osteoarthritis and trigger osteoarthritic changes in mice. Ann Rheum Dis 71:1249–1253
Ahn DK, Lee S, Moon SH, Boo KH, Chang BK, Lee JI (2014) Ossification of the ligamentum flavum. Asian Spine J 8:89–96
Han Y, Hong Y, Li L, Li T, Zhang Z, Wang J, Xia H, Tang Y, Shi Z, Han X, Chen T, Liu Q, Zhang M, Zhang K, Hong W, Xue Y (2018) A transcriptome-level study identifies changing expression profiles for ossification of the ligamentum flavum of the spine. Mol Therapy Nucleic Acids 12:872–883
Yang X, Qu X, Meng X, Li M, Fan D, Fan T, Huang AY, Chen Z, Zhang C (2018) MiR-490-3p inhibits osteogenic differentiation in thoracic ligamentum flavum cells by targeting FOXO1. Int J Biol Sci 14:1457–1465
Yayama T, Mori K, Okumura N, Nishizawa K, Kumagai K, Nakamura A, Imai S (2018) Wnt signaling pathway correlates with ossification of the spinal ligament: a microRNA array and immunohistochemical study. J Orthopaedic Sci 23:26–31
Li XC, Jin F, Wang BY, Yin XJ, Hong W, Tian FJ (2019) The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics 9:3853–3865
Takahashi T, Hanakita J, Minami M (2018) Pathophysiology of calcification and ossification of the ligamentum flavum in the cervical spine. Neurosurg Clin N Am 29:47–54
Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG (2015) Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine 40:E675–693
Zhang C, Zhang M, Ge S, Huang W, Lin X, Gao J, Gong J, Shen L (2019) Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K-Akt signaling in gastric cancer. Cancer Med. https://doi.org/10.1002/cam4.2360
Liu J, Eckert MA (2018) m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell 20:1074–1083
Funding
Funding was provided by Natural Science Foundation of Shandong Province (Grant No. ZR2017MH120).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hai-Feng Wang, Ming-jie Kuang, Shi-jie Han, An-bang Wang, Jie Qiu, Feng Wang, Bing-yi Tan and Da-Chuan Wang declare that there is no conflict of interest.
Human and Animal Rights and Informed Consent
The human surgical procedures and sampling were approved by the Human Ethics Committee of Provincial Hospital Affiliated to Shandong University. The Animal Care and Use Committee of Provincial Hospital Affiliated to Shandong University approved the animal procedures and experimentation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, HF., Kuang, Mj., Han, Sj. et al. BMP2 Modified by the m6A Demethylation Enzyme ALKBH5 in the Ossification of the Ligamentum Flavum Through the AKT Signaling Pathway. Calcif Tissue Int 106, 486–493 (2020). https://doi.org/10.1007/s00223-019-00654-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00654-6