Abstract
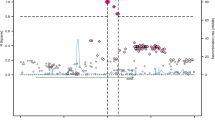
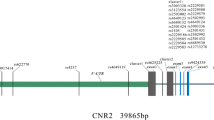
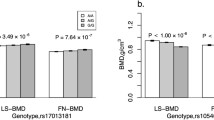
As one of the most common types of osteoporosis, postmenopausal osteoporosis (PMOP) is caused by both genetic and environmental factors. Previous studies have indicated that SOX9 activity is tightly regulated to ensure normal bone mineral density (BMD) in the adult skeleton, and the COL9A1 promoter region can be transactivated by SOX9. In this study, we aimed to investigate the potential association between PMOP and the COL9A1 and SOX9 genes. A total of 10,443 postmenopausal women, including 2288 patients and 3557 controls in the discovery stage and 1566 patients and 3032 controls in the validation stage, were recruited. Forty-three tag SNPs (36 in COL9A1 and 7 in SOX9) were selected for genotyping to evaluate the association of the SOX9 gene with PMOP and BMD. Association and bioinformatics analyses were performed for PMOP. BMD and serum level of SOX9 were also utilized as quantitative phenotypes in further analyses. SNP rs73354570 of SOX9 was significantly associated with PMOP in both discovery stages (OR 1.24 [1.10–1.39], P = 3.56 × 10−4, χ2 = 12.75) and combined samples (OR 1.25 [1.15–1.37], P = 5.25 × 10−7, χ2 = 25.17). Further analyses showed that the SNP was also significantly associated with BMD and serum levels of the SOX9 protein. Our results provide further supportive evidence for the association of the SOX9 gene with PMOP and of the SOX9 gene with the variation of BMD in postmenopausal Han Chinese women. This study supports a role for SOX9 in the etiology of PMOP, adding to the current understanding of the susceptibility of osteoporosis.

Similar content being viewed by others
References
Parfitt AM (1982) The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res 4:1–6
Appelman-Dijkstra NM, Papapoulos SE (2015) Modulating bone resorption and bone formation in opposite directions in the treatment of postmenopausal osteoporosis. Drugs 75:1049–1058
Soen S, Fukunaga M, Sugimoto T et al (2013) Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31:247–257
Willson T, Nelson SD, Newbold J, Nelson RE, LaFleur J (2015) The clinical epidemiology of male osteoporosis: a review of the recent literature. Clin Epidemiol 7:65–76
Slemenda CW, Christian JC, Williams CJ et al (1991) Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res 6(6):561–567
Flicker L, Hopper JL, Rodgers L et al (1995) Bone density determinants in elderly women: a twin study. J Bone Miner Res 10(11):1607–1613
Hernandez-de Sosa N, Athanasiadis G, Malouf J et al (2014) Heritability of bone mineral density in a multivariate family-based study. Calcif Tissue Int 94(6):590–596
Shang M, Lin L, Cui H (2013) Association of genetic polymorphisms of RANK, RANKL and OPG with bone mineral density in Chinese peri-and postmenopausal women. Clin Biochem 46(15):1493–1501
Bandres E, Pombo I, Gonzalez-Huarriz M et al (2005) Association between bone mineral density and polymorphisms of the VDR, ERα, COL1A1 and CTR genes in Spanish postmenopausal women. J Endocrinol Investig 28(6):312–321
Morris JA, Kemp JP, Youlten SE et al (2019) An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet 51(2):258
Estrada K, Styrkarsdottir U, Evangelou E et al (2012) Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 44:491–501
Kemp JP, Morris JA, Medina-Gomez C et al (2017) Identification of 153 new loci associated with heel bone mineral density and functional involvement of GPC6 in osteoporosis. Nat Genet 49:1468–1475
Mafi Golchin M, Heidari L, Ghaderian SM, Akhavan-Niaki H (2016) Osteoporosis: a silent disease with complex genetic contribution. J Genet Genomics 43:49–61
Wang CJ, Iida K, Egusa H, Hokugo A, Jewett A, Nishimura I (2008) Trabecular bone deterioration in col9a1+/- mice associated with enlarged osteoclasts adhered to collagen IX-deficient bone. J Bone Miner Res 23:837–849
Parsons P, Gilbert SJ, Vaughan-Thomas A, Sorrell DA, Notman R, Bishop M, Hayes AJ, Mason DJ, Duance VC (2011) Type IX collagen interacts with fibronectin providing an important molecular bridge in articular cartilage. J Biol Chem 286:34986–34997
Brachvogel B, Zaucke F, Dave K, Norris EL, Stermann J, Dayakli M, Koch M, Gorman JJ, Bateman JF, Wilson R (2013) Comparative proteomic analysis of normal and collagen IX null mouse cartilage reveals altered extracellular matrix composition and novel components of the collagen IX interactome. J Biol Chem 288:13481–13492
Posey KL, Hankenson K, Veerisetty AC, Bornstein P, Lawler J, Hecht JT (2008) Skeletal abnormalities in mice lacking extracellular matrix proteins, thrombospondin-1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am J Pathol 172:1664–1674
Mustafa Z, Chapman K, Irven C et al (2000) Linkage analysis of candidate genes as susceptibility loci for osteoarthritis—suggestive linkage of COL9A1 to female hip osteoarthritis. Rheumatology 39(3):299–306
Shi X, Zhang F, Lv A et al (2015) COL9A1 gene polymorphism is associated with Kashin-Beck disease in a northwest Chinese Han population. PLoS ONE 10(3):e0120365
Akiyama H (2008) Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol 18:213–219
Takeda K, Kou I, Otomo N et al (2019) A multiethnic meta-analysis defined the association of rs12946942 with severe adolescent idiopathic scoliosis. J Hum Genet 64(5):493
Miyake A, Kou I, Takahashi Y et al (2013) Identification of a susceptibility locus for severe adolescent idiopathic scoliosis on chromosome 17q24.3. PLoS ONE 8(9):e72802
Roosenboom J, Lee MK, Hecht JT et al (2018) Mapping genetic variants for cranial vault shape in humans. PLoS ONE 13(4):e0196148
Baird DA, Evans DS, Kamanu FK et al (2019) Identification of Novel loci associated with hip shape: a meta-analysis of genomewide association studies. J Bone Miner Res 34(2):241–251
Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B (2001) Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci USA 98:6698–6703
Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B (2002) The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16(21):2813–2828
Wang Y, Zhang X, Niu X et al (2019) The genetic relationship of SOX9 polymorphisms with osteoarthritis risk in Chinese population: a case-control study. Medicine 98(8):e14096
Song Y, Du Z, Ren M et al (2016) Significant associations of SOX9 gene polymorphism and gene expression with the risk of osteonecrosis of the femoral head in a Han population in Northern China. BioMed Res Int. https://doi.org/10.1155/2016/5695317
Liang B, Cotter MM, Chen D, Hernandez CJ, Zhou G (2012) Ectopic expression of SOX9 in osteoblasts alters bone mechanical properties. Calcif Tissue Int 90:76–89
Zhang L, Choi HJ, Estrada K et al (2013) Multistage genome-wide association meta-analyses identified two new loci for bone mineral density. Hum Mol Genet 23(7):1923–1933
Qin L, Liu Y, Wang Y, Wu G, Chen J, Ye W, Yang J, Huang Q (2016) Computational characterization of osteoporosis associated SNPs and genes identified by genome-wide association studies. PLoS ONE 11:e0150070
Zhang P, Jimenez SA, Stokes DG (2003) Regulation of human COL9A1 gene expression. Activation of the proximal promoter region by SOX9. J Biol Chem 278:117–123
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4:7
Xie D, Boyle AP, Wu L, Zhai J, Kawli T, Snyder M (2013) Dynamic trans-acting factor colocalization in human cells. Cell 155:713–724
Consortium GT (2013) The genotype-tissue expression (GTEx) project. Nat Genet 45:580–585
Zhou G, Zheng Q, Engin F, Munivez E, Chen Y, Sebald E, Krakow D, Lee B (2006) Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad Sci USA 103:19004–19009
Song Y, Du Z, Ren M, Yang Q, Sui Y, Wang Q, Wang A, Zhao H, Wang J, Zhang G (2016) Significant associations of SOX9 gene polymorphism and gene expression with the risk of osteonecrosis of the femoral head in a han population in Northern China. BioMed Res Int 2016:5695317
Hattori T, Muller C, Gebhard S et al (2010) SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 137:901–911
Jensen ED, Nair AK, Westendorf JJ (2007) Histone deacetylase co-repressor complex control of Runx2 and bone formation. Crit Rev Eukaryot Gene Expr 17:187–196
Xiao Z, Awad HA, Liu S, Mahlios J, Zhang S, Guilak F, Mayo MS, Quarles LD (2005) Selective Runx2-II deficiency leads to low-turnover osteopenia in adult mice. Dev Biol 283:345–356
Guan F, Zhang B, Yan T, Li L, Liu F, Li T, Feng Z, Zhang B, Liu X, Li S (2014) MIR137 gene and target gene CACNA1C of miR-137 contribute to schizophrenia susceptibility in Han Chinese. Schizophr Res 152(1):97–104
Jia X, Zhang T, Li L, Fu D, Lin H, Chen G, Liu X, Guan F (2016) Two-stage additional evidence support association of common variants in the HDAC3 with the increasing risk of schizophrenia susceptibility. Am J Med Genet B 171(8):1105–1111
Zhang T, Zhu L, Ni T, Liu D, Chen G, Yan Z, Lin H, Guan F, Rice JP (2018) Voltage-gated calcium channel activity and complex related genes and schizophrenia: A systematic investigation based on Han Chinese population. J Psychiatr Res 106:99–105
Han W, Zhang T, Ni T, Zhu L, Liu D, Chen G, Lin H, Chen T, Guan F (2019) Relationship of common variants in CHRNA5 with early-onset schizophrenia and executive function. Schizophr Res 206:407–412
Liu X, Hou Y, Yan T, Guo Y, Han W, Guan F, Chen T, Li T (2014) Dopamine D3 receptor-regulated NR2B subunits of N-methyl-d-aspartate receptors in the nucleus accumbens involves in morphine-induced locomotor activity. CNS Neurosci Ther 20(9):823–829
Zhu L, Li J, Dong N, Guan F, Liu Y, Ma D, Goh EL, Chen T (2016) mRNA changes in nucleus accumbens related to methamphetamine addiction in mice. Sci Rep 6:36993
Li J, Zhu L, Guan F, Yan Z, Liu D, Han W, Chen T (2018) Relationship between schizophrenia and changes in the expression of the long non-coding RNAs Meg3, Miat, Neat1 and Neat2. J Psychiatr Res 106:22–30
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Jun Lu and Hongliang Liu. Performed the experiments: Hongliang Liu and Hongmou Zhao. Analyzed the data: Hua Lin. Contributed reagents / materials / analysis tools: Zhong Li, Hanzhong Xue and Yunzhi Zhang. Wrote the paper: Hongliang Liu.
Corresponding author
Ethics declarations
Conflict of interest
Hongliang Liu, Hongmou Zhao, Hua Lin, Zhong Li, Hanzhong Xue, Yunzhi Zhang and Jun Lu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This study was performed in accordance with the ethical guidelines of the Helsinki Declaration of 1975 (revised in 2008) and was approved by the Medical Ethics Committee of Honghui Hospital of Xi’an Jiaotong University. Informed consent was obtained from all subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Zhao, H., Lin, H. et al. Relationship of COL9A1 and SOX9 Genes with Genetic Susceptibility of Postmenopausal Osteoporosis. Calcif Tissue Int 106, 248–255 (2020). https://doi.org/10.1007/s00223-019-00629-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00629-7