Abstract
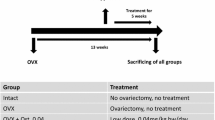
Non-steroidal selective androgen receptor modulators, including ostarine, have been developed as an alternative to steroidal hormones. Ostarine has shown a beneficial effect on bone in experimental studies, but no data regarding the effect of ostarine on bone healing have yet been reported. We investigated effects of ostarine on bone healing in ovariectomized rats. Sprague-Dawley rats (3 months old) were ovariectomized (Ovx, n = 46) or left intact (Non-Ovx, n = 10). After 8 weeks, an osteotomy of the tibia metaphysis was created in all rats, and the Ovx rats were divided into four groups: untreated Ovx (n = 10) and three Ovx groups (each of 12 rats) treated with ostarine at doses of 0.04, 0.4, or 4 mg/kg BW (OS-0.04, OS-0.4, and OS-4 groups). Five weeks later, bone healing was analyzed. The OS-4 dose enhanced callus formation, increased callus density, accelerated bridging time of the osteotomy, and elevated alkaline phosphatase gene expression in callus and its protein expression in serum. In the Ovx group, most of the callus parameters were diminished. All OS treatments increased the weight of the gastrocnemius muscle, but only partly enhanced uterus weight in OS-0.4 and OS-4. Serum cholesterol level was reduced, and serum phosphorus was elevated in OS-0.04 and OS-4. Ostarine appeared to have a positive effect on early bone healing in ovariectomized rats. Considering its favorable effect on non-osteotomized bone and muscle, this treatment could be further explored as a therapy for osteoporosis. However, possible metabolic side effects should first be evaluated.





Similar content being viewed by others
References
Carrascosa A, Audi L, Ferrandez MA, Ballabriga A (1990) Biological effects of androgens and identification of specific dihydrotestosterone-binding sites in cultured human fetal epiphyseal chondrocytes. J Clin Endocrinol Metab 70:134–140
Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE (1997) The localization of androgen receptors in human bone. J Clin Endocrinol Metab 82:3493–3497
Sinnesael M, Claessens F, Laurent M, Dubois V, Boonen S, Deboel L, Vanderschueren D (2012) Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res 27:2535–2543
Määttä JA, Büki KG, Ivaska KK, Nieminen-Pihala V, Elo TD, Kähkönen T, Poutanen M, Härkönen P, Väänänen K (2013) Inactivation of the androgen receptor in bone-forming cells leads to trabecular bone loss in adult female mice. BoneKey Rep 2:440. https://doi.org/10.1038/bonekey.2013.174
Sinnesael M, Jardi F, Deboel L, Laurent MR, Dubois V, Zajac JD, Davey RA et al (2015) The androgen receptor has no direct antiresorptive actions in mouse osteoclasts. Mol Cell Endocrinol 411:198–206
Lee JS, LaCroix AZ, Wu L, Cauley JA, Jackson RD, Kooperberg C, Leboff MS, Robbins J et al (2008) Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. J Clin Endocrinol Metab 93:1796–1803
Cauley JA, Danielson ME, Jammy GR, Bauer DC, Jackson R, Wactawski-Wende J, Chlebowski RT et al (2017) Sex steroid hormones and fracture in a multiethnic cohort of women: the women’s health initiative study (WHI). J Clin Endocrinol Metab 102:1538–1547
Gruenwald DA, Matsumoto AM (2003) Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriat Soc 51:101–115
Davis SR, Wahlin-Jacobsen S (2015) Testosterone in women-the clinical significance. Lancet Diabetes Endocrinol 3:980–992
Zhang X, Lanter JC, Sui Z (2009) Recent advances in the development of selective androgen receptor modulators. Expert Opin Ther Patents 19:1239–1258
Narayanan R, Coss CC, Dalton JT (2018) Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol 465:134–142
Omwancha J, Brown TR (2006) Selective androgen receptor modulators: in pursuit of tissue-selective androgens. Curr Opin Invest Drugs 7:873–881
Segal S, Narayanan R, Dalton JT (2006) Therapeutic potential of the SARMs: revisiting the androgen receptor for drug discovery. Expert Opin Invest Drugs 15:377–387
Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST et al (2011) The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle 2:153–161
Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, Johnston MA, Steiner MS (2013) Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol 14:335–345
Crawford J, Prado CM, Johnston MA, Gralla RJ, Taylor RP, Hancock ML, Dalton JT et al (2016) Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER Trials). Curr Oncol Rep 18:37. https://doi.org/10.1007/s11912-016-0522-0
Kearbey JD, Gao W, Narayanan R, Fisher SJ, Wu D, Miller DD, Dalton JT (2007) Selective androgen receptor modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharm Res 24:328–335
Hoffmann DB, Komrakova M, Pflug S, von Oertzen M, Saul D, Weiser L, Walde TA et al (2018) Evaluation of ostarine as a selective androgen receptor modulator in a rat model of postmenopausal osteoporosis. J Bone Miner Metab. https://doi.org/10.1007/s00774-018-0929-9
WADA (World Anti-Doping Agency). https://www.wada-ama.org/en/content/what-is-prohibited/prohibited-at-all-times/anabolic-agents. Accessed 30 Jan 2019
Komrakova M, Sehmisch S, Tezval M, Schmelz U, Frauendorf H, Grueger T et al (2011) Impact of 4-methylbenzylidene camphor, daidzein, and estrogen on intact and osteotomized bone in osteopenic rats. J Endocrinol 211:157–168
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15:175–192
Stuermer EK, Sehmisch S, Rack T, Wenda E, Seidlova-Wuttke D, Tezval M et al (2010) Estrogen and raloxifene improve metaphyseal fracture healing in the early phase of osteoporosis. A new fracture-healing model at the tibia in rat. Langenbecks Arch Surg 395:163–172
Komrakova M, Fiebig J, Hoffmann DB, Krischek C, Lehmann W, Stuermer KM, Sehmisch S (2018) The advantages of bilateral osteotomy over unilateral osteotomy for osteoporotic bone healing. Calcif Tissue Int 103:80–94
Komrakova M, Weidemann A, Dullin C, Ebert J, Tezval M, Stuermer KM et al (2015) The impact of strontium ranelate on metaphyseal bone healing in ovariectomized rats. Calcif Tissue Int 97:391–401
Komrakova M, Hoffmann DB, Nuehnen V, Stueber H, Wassmann M, Wicke M et al (2016) The effect of vibration treatments combined with teriparatide or strontium ranelate on bone healing and muscle in ovariectomized rats. Calcif Tissue Int 99:408–422
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. JBMR 25:1468–1486
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Saul D, Harlas B, Ahrabi A, Kosinsky RL, Hoffmann DB, Wassmann N, Wigger R et al (2018) Effect of strontium ranelate on the muscle and vertebrae of ovariectomized rats. Calcif Tissue Int 102:705–719
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T (2001) Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 28:80–86
Hao YJ, Zhang G, Wang YS, Qin L, Hung WY, Leung K, Pei FX (2007) Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 41:631–638
Steiner M, Volkheimer D, Meyers N, Wehner T, Wilke HJ, Claes L, Ignatius A (2015) Comparison between different methods for biomechanical assessment of ex vivo fracture callus stiffness in small animal bone healing studies. PLoS ONE 10(3):e0119603. https://doi.org/10.1371/journal.pone.0119603
Cheng B-H, Chu T-MG, Chang C, Kang H-Y, Huang K-E (2013) Testosterone delivered with a xcaffold is as effective as bone morphologic protein-2 in promoting the repair of critical-size segmental defect of femoral bone in mice. PLoS ONE 8(8):e70234. https://doi.org/10.1371/journal.pone.0070234
Gesicki M, Tibba J, Nguyen CK, Beil FT, Rueger JM, Haberland M, Amling M (2003) Testosterone is a potent accelerator of fracture healing: early structural reconstruction and improved biomechanical stability. Osteo Trauma Care 11:3–5
Wiren KM, Chapman Evans A, Zhang XW (2002) Osteoblast differentiation influences androgen and estrogen receptor-alpha and -beta expression. J Endocrinol 175:683–694
Mohamad N-V, Soelaiman I-N, Chin K-Y (2016) A concise review of testosterone and bone health. Clin Interv Aging 11:1317–1324
Aro HT, Kulkova J, Moritz N, Kähkönen E, Mattila RH (2015) Local delivery of a selective androgen receptor modulator failed as an anabolic agent in a rat bone marrow ablation model. Acta Orthop 86:751–759
Harry LE, Sandison A, Paleolog EM, Hansen U, Pearse MF, Nanchahal J (2008) Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res 26:1238–1244
Girgis CM, Mokbel N, DiGirolamo DJ (2014) Therapies for musculoskeletal disease: can we treat two birds with one stone? Curr Osteoporos Rep. 12:142–153
Garber K (2016) No longer going to waste. Nat Biotechnol 34:458–461
Alatalo SL, Peng Z, Janckila AJ, Kaija H, Vihko P, Vaananen HK, Halleen JM (2003) A novel immunoassay for the determination of tartrate-resistant acid phosphatase 5b from rat serum. J Bone Miner Res 18:134–139
Rissanen JP, Suominen MI, Peng Z, Halleen JM (2008) Secreted tartrate-resistant acid phosphatase 5b is a marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int 82:108–115
Schell H, Lienau J, Epari DR, Seebeck P, Exner C, Muchow S, Bragulla H, Haas NP, Duda GN (2006) Osteoclastic activity begins early and increases over the course of bone healing. Bone 38:547–554
Sinnesael M, Jardi F, Deboel L, Laurent MR, Dubois V, Zajac JD, Davey RA, Carmeliet G, Claessens F, Vanderschueren D (2015) The androgen receptor has no direct antiresorptive actions in mouse osteoclasts. Mol Cell Endocrinol 411:198–206
Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, Börjesson AE, Ohlsson C (2014) Sex steroid actions in male bone. Endocr Rev 35:906–960
NAMS Position Statement (2005) The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause 12:497–511
Simitsidellis I, Kelepouri O, Gibson DA, Esnal-Zufiaurre A, Saunders PTK (2018) Androgen modulation of mouse uterus: a tissue-based bioassay for testing endogenous and synthetic androgen receptor modulators (SARMs). Endocrine Abstracts. https://doi.org/10.1530/endoabs.59.oc5.5
Acknowledgements
The present study was funded by the German Research Foundation (DFG SE 1966/6-1, KO 4646/3-1). The authors are grateful to R. Castro-Machguth and A. Witt for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Marina Komrakova, Judith Furtwängler, Daniel Bernd Hoffmann, Wolfgang Lehmann, Arndt Friedrich Schilling, and Stephan Sehmisch declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The animal study protocol was approved by the local regional government (14/1396, Oldenburg, Germany) in accordance with German animal protection laws prior to performing the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Komrakova, M., Furtwängler, J., Hoffmann, D.B. et al. The Selective Androgen Receptor Modulator Ostarine Improves Bone Healing in Ovariectomized Rats. Calcif Tissue Int 106, 147–157 (2020). https://doi.org/10.1007/s00223-019-00613-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00613-1