Abstract
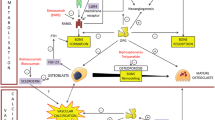
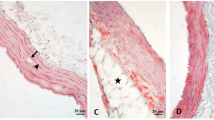
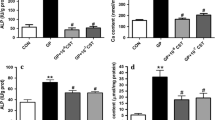
Low circulating levels of undercarboxylated osteocalcin (ucOC) is associated with a higher risk of cardiovascular disease, yet whether ucOC has a direct effect on endothelium-dependent vasorelaxation, or in proximity to its postulated receptor, the class CG protein-coupled receptor (GPCR6A), in blood vessels remains unclear. Immunohistochemistry and proximity ligation assays were used to localize the presence of ucOC and GPRC6A and to determine the physical proximity (< 40 nm) in radial artery segments collected from patients undergoing coronary artery bypass surgery (n = 6) which exhibited calcification (determined by Von Kossa) and aorta from New Zealand white rabbits exhibiting atherosclerotic plaques. Endothelium-dependent vasorelaxation was assessed using cumulative doses of acetylcholine in vitro on abdominal aorta of rabbits fed a normal chow diet (n = 10) and a 4-week atherogenic diet (n = 9) pre-incubated with ucOC (10 ng/mL) or vehicle. Both ucOC and GPRC6A were localized in human and rabbit diseased-blood vessels. Proximity ligation assay staining demonstrated physical proximity of ucOC with GPRC6A only within plaques in rabbit arteries and the endothelium layer of rabbit arterioles. Endothelium-dependent vasorelaxation was impaired in atherogenic abdominal aorta compared to healthy aorta and ucOC attenuated this impairment. ucOC attenuated impaired endothelium-dependent vasorelaxation in rabbit abdominal aorta following an atherogenic diet, however, this effect may be independent of GPRC6A. It is important that future studies determine the underlying cellular mechanisms by which ucOC effects blood vessels as well as whether it can be used as a therapeutic agent against the progression of atherosclerosis.








Similar content being viewed by others
References
Creager MA et al (2003) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 108(12):1527–1532
AIHW (2014) Cardiovascular disease, diabetes and chronic kidney disease: Australian facts: prevalence and incidence. Cardiovascular, diabetes and chronic kidney disease series no. 2. Cat. no. CDK 2. AIHW, Canberra
Heidenreich PA et al (2011) Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123(8):933–944
Prashar Y et al (2017) Emerging role of various signaling pathways in the pathogenesis and therapeutics of atherosclerosis. Rev Vasc Med 10–11:1–12
Rached MT et al (2010) FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest 120(1):357–368
Ferron M et al (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142(2):296–308
Levinger I et al (2016) The effects of muscle contraction and recombinant osteocalcin on insulin sensitivity ex vivo. Osteoporos Int 27(2):653–663
Lee NK et al (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130(3):456–469
Oury F et al (2011) Endocrine regulation of male fertility by the skeleton. Cell 144(5):796–809
Pi M, Quarles LD (2012) Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology 153(5):2062–2069
Lin X et al (2016) Hindlimb immobilization, but not castration, induces reduction of undercarboxylated osteocalcin associated with muscle atrophy in rats. J Bone Miner Res 31(11):1967–1978
Levinger I et al (2014) The effect of acute exercise on undercarboxylated osteocalcin and insulin sensitivity in obese men. J Bone Miner Res 29(12):2571–2576
Levinger I et al (2011) The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporos Int 22(5):1621–1626
Hwang YC et al (2009) The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev 25(8):768–772
Kanazawa I et al (2011) Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int 22(1):187–194
Kim KM et al (2016) Lower uncarboxylated osteocalcin and higher sclerostin levels are significantly associated with coronary artery disease. Bone 83:178–183
Zhang M et al (2015) Undercarboxylated osteocalcin as a biomarker of subclinical atherosclerosis in non-dialysis patients with chronic kidney disease. J Biomed Sci 22:75
Confavreux CB et al (2013) Higher serum osteocalcin is associated with lower abdominal aortic calcification progression and longer 10-year survival in elderly men of the MINOS cohort. J Clin Endocrinol Metab 98(3):1084–1092
Okura T et al (2010) Undercarboxylated osteocalcin is a biomarker of carotid calcification in patients with essential hypertension. Kidney Blood Press Res 33(1):66–71
Zulli A et al (2008) Human diseased arteries contain cells expressing leukocytic and embryonic stem cell markers. Hum Pathol 39(5):657–665
Zulli A et al (2003) The resistance of the IMA to atherosclerosis might be associated with its higher eNOS, ACE and ET-A receptor immunoreactivity. Arterioscler Thromb Vasc Biol 23(7):1308
Zulli A et al (2008) Co-localization of angiotensin-converting enzyme 2-, octomer-4- and CD34-positive cells in rabbit atherosclerotic plaques. Exp Physiol 93(5):564–569
Liao J, Huang W, Liu G (2015) Animal models of coronary heart disease. J Biomed Res 30(1):3–10
Tsukada T et al (1987) HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol 126(1):51–60
Bagchi S, Fredriksson R, Wallén-Mackenzie Å (2015) In situ proximity ligation assay (PLA). In: Hnasko R (ed) ELISA: methods and protocols. Springer, New York, NY, pp 149–159
Karsenty G (1998) Transcriptional regulation of osteoblast differentiation during development. Front Biosci 3:d834–d837
Ducy P (2011) The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 54(6):1291–1297
Kapustin AN, Shanahan CM (2011) Osteocalcin: a novel vascular metabolic and osteoinductive factor? Arterioscler Thromb Vasc Biol 31(10):2169–2171
Idelevich A, Rais Y, Monsonego-Ornan E (2011) Bone Gla protein increases HIF-1alpha-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol 31(9):e55–e71
Sheng L et al (2013) Serum osteocalcin level and its association with carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol 12:22
Zhang H et al (2015) Correlation between osteocalcin-positive endothelial progenitor cells and spotty calcification in patients with coronary artery disease. Clin Exp Pharmacol Physiol 42(7):734–739
Ferron M et al (2008) Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci 105(13):5266–5270
Ferron M et al (2012) Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 50:568–575
Levinger I et al (2017) Multifaceted interaction of bone, muscle, lifestyle interventions and metabolic and cardiovascular disease: role of osteocalcin. Osteoporos Int 28(8):2265–2273
Tacey A et al (2018) Potential role for osteocalcin in the development of atherosclerosis and blood vessel disease. Nutrients 10(10):1426
Corselli M et al (2012) The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21(8):1299–1308
Hass R et al (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal: CCS 9:12
Paiva AE et al (2017) Endothelial cells as precursors for osteoblasts in the metastatic prostate cancer bone. Neoplasia (New York) 19(11):928–931
Pi M et al (2005) Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280(48):40201–40209
Pi M, Wu Y, Quarles LD (2011) GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res 26(7):1680–1683
Lin X et al (2017) Recombinant uncarboxylated osteocalcin per se enhances mouse skeletal muscle glucose uptake in both extensor digitorum longus and soleus muscles. Front Endocrinol (Lausanne) 8:330
Sun L, Ye RD (2012) Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin 33(3):342–350
Tousoulis D et al (2012) The role of nitric oxide on endothelial function. Curr Vasc Pharmacol 10(1):4–18
Mudau M et al (2012) Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Africa 23(4):222–231
Kondo A, Kawakubo-Yasukochi TK, Mizokami A, Chishaki S, Takeuchi H, Hirata H (2016) Uncarboxylated osteocalcin increases serum nitric oxide levels and ameliorates hypercholesterolemia in mice fed an atherogenic diet. Electron J Biol 13(1):22–28
Chistiakov DA, Orekhov AN, Bobryshev YV (2015) Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol 6:365
Millar SA, Anderson SI, O’Sullivan E (2019) Human vascular cell responses to the circulating bone hormone osteocalcin. J Cell Physiol 234(11):21039–21048
Faure H et al (2009) Molecular determinants of non-competitive antagonist binding to the mouse GPRC6A receptor. Cell Calcium 46(5–6):323–332
Clemmensen C et al (2014) The GPCR, class C, group 6, subtype A (GPRC6A) receptor: from cloning to physiological function. Br J Pharmacol 171(5):1129–1141
O’Connor EM, Durack E (2017) Osteocalcin: the extra-skeletal role of a vitamin K-dependent protein in glucose metabolism. J Nutr Intermed Metab 7:8–13
Schurgers LJ et al (2018) Initiation and propagation of vascular calcification is regulated by a concert of platelet- and smooth muscle cell-derived extracellular vesicles. Front Cardiovasc Med 5:36
Durham AL et al (2018) Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res 114(4):590–600
Bostrom K et al (1993) Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 91(4):1800–1809
Montezano AC et al (2010) Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 56(3):453–462
Acknowledgements
Associate Professor Itamar Levinger was supported by a Heart Foundation Future Leader Fellow (ID: 100040). This study was funded by The Rebecca L Cooper Medical Research Foundation.
Author information
Authors and Affiliations
Contributions
TQ, LKG: data curation, formal analysis, investigation & writing drafts. LKG and ABT: writing-review & editing. DLH: resources & writing-review & editing. BFB: resources & writing-review & editing. VA: writing-review & editing and supervision. IL: funding acquisition, resources, supervision and writing-review & editing. AZ: conceptualization, investigation, methodology, resources, software, supervision and writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
Tawar Qaradakhi, Laura K. Gadanec, Alex B. Tacey, David L. Hare, Brian F. Buxton, Vasso Apostoloppoulos, Itamar Levinger and Anthony Zulli declare that they have no conflict of interest. These authors also declare no competing financial interests.
Ethical Approval
The study was approved by the Austin Hospital Medical Research Ethics Committee and followed institutional guidelines that conform with the Declaration of Helsinki.
Human and Animal Rights and Informed Consent
Patients gave informed consent to collect discarded arteries.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qaradakhi, T., Gadanec, L.K., Tacey, A.B. et al. The Effect of Recombinant Undercarboxylated Osteocalcin on Endothelial Dysfunction. Calcif Tissue Int 105, 546–556 (2019). https://doi.org/10.1007/s00223-019-00600-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00600-6