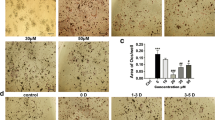
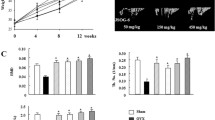
Abstract
Anti-resorptive agents like bisphosphonates have been widely used for the treatment of postmenopausal osteoporosis. However, their long-term safety and efficacy are still controversial. This study is to examine the effect of Asiatic acid (AA) in osteoclastic differentiation, and further to investigate its effect on bone quality in animals. Effect of AA on osteoclastic differentiation was measured by Tartrate-resistant acid phosphatase stain, bone resorption pit assays, and quantitative real-time polymerase chain reaction. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and transforming growth factor-β (TGF-β) signaling were measured by western blot before and after AA treatment. Ovariectomized (OVX) wild-type or Smad7 partially knock out mice were used to evaluate the effects of AA on bone quality by micro-computed tomography, mechanical test, and histomorphometry. Results revealed a dose-dependent inhibitory effect of AA on osteoclastic differentiation. After AA treatment, Smad7 was upregulated, while NF-κB and TGF-β signaling were inhibited during osteoclastic differentiation. Results from animal study revealed that AA prevented bone from further loss caused by OVX and increased the mechanical properties of femur in wild-type animals. AA also prevented bone loss in the Smad7-deficient animals. When combining with OVX in the Smad7-deficient mice, AA could only partially preserve their bone mass. Taken together, we found that AA effectively inhibited osteoclastic differentiation and attenuated osteoporosis, which effects may be through TGF-β and NF-κB pathways. This study reveals that AA may be a potential anti-resorptive agent for postmenopausal osteoporosis.







Similar content being viewed by others
References
Curtis AEM, Moon ARJ, Dennison BEM et al (2015) Recent advances in the pathogenesis and treatment of osteoporosis. Clin Med (Northfield Il) 15:92–96
Khosla S, Hofbauer LC (2017) Osteoporosis treatment : recent developments and ongoing challenges. Lancet Diabetes Endocrinol 5:898–907. https://doi.org/10.1016/S2213-8587(17)30188-2
Whitaker M, Guo J, Ph D et al (2012) Bisphosphonates for Osteoporosis—where do we go. New Engl J Med 366:2048–2051
Black DM, Bauer DC, Schwartz AV et al (2012) Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? New Engl J Med 366:2051–2053
Hald JD, Evangelou E, Langdahl BL, Ralston SH (2015) Bisphosphonates for the prevention of fractures in osteogenesis imperfecta: meta-analysis of placebo-controlled trials. J Bone Miner Res 30:929–933. https://doi.org/10.1002/jbmr.2410
Feng X, McDonald JM (2011) Disorders of bone remodeling. Annu Rev Pathol Mech Dis 6:121–145. https://doi.org/10.1146/annurev-pathol-011110-130203
Wu M, Chen G, Li Y (2016) TGF- β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4:16009. https://doi.org/10.1038/boneres.2016.9
Crane JL, Xian L, Cao X (2016) Role of TGF-β signaling in coupling bone remodeling. In: Feng X-H, Xu P, Lin X (eds) Methods in molecular biology. Springer, New York, pp 287–300
Tang L, He R, Yang G et al (2012) Asiatic acid inhibits liver fibrosis by blocking TGF-beta/Smad signaling in vivo and in vitro. PLoS ONE 7:e31350. https://doi.org/10.1371/journal.pone.0031350
Iwai T, Murai J, Yoshikawa H, Tsumaki N (2008) Smad7 inhibits chondrocyte differentiation at multiple steps during endochondral bone formation and down-regulates p38 MAPK pathways. J Biol Chem 283:27154–27164. https://doi.org/10.1074/jbc.M801175200
Kawakatsu M, Kanno S, Gui T et al (2011) Loss of Smad3 gives rise to poor soft callus formation and accelerates early fracture healing. Exp Mol Pathol 90:107–115. https://doi.org/10.1016/j.yexmp.2010.10.011
Yasui T, Kadono Y, Nakamura M et al (2011) J BMR regulation of RANKL-induced osteoclastogenesis by TGF-b through molecular interaction between. J Bone Miner Res 26:1447–1456. https://doi.org/10.1002/jbmr.357
Li N, Lee WYW, Lin S et al (2014) Partial loss of Smad7 function impairs bone remodeling, osteogenesis and enhances osteoclastogenesis in mice. Bone 67:46–55. https://doi.org/10.1016/j.bone.2014.06.033
Luo J, Yang Z, Ma Y et al (2016) LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med 22:539–546. https://doi.org/10.1038/nm.4076
Lee WY, Li N, Lin S et al (2016) miRNA-29b improves bone healing in mouse fracture model. Mol Cell Endocrinol 430:97–107. https://doi.org/10.1016/j.mce.2016.04.014
Huang S, Xu L, Sun Y et al (2015) An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. J Orthop Transl 3:26–33. https://doi.org/10.1016/j.jot.2014.07.005
Zalli D, Neff L, Nagano K et al (2016) The actin-binding protein cofilin and its interaction with cortactin are required for podosome patterning in osteoclasts and bone resorption in vivo and in vitro. J Bone Miner Res 31:1701–1712. https://doi.org/10.1002/jbmr.2851
Lin S, Lee WYW, Huang M et al (2016) Aspirin prevents bone loss with little mechanical improvement in high-fat-fed ovariectomized rats. Eur J Pharmacol 791:331–338. https://doi.org/10.1016/j.ejphar.2016.09.018
Lin S, Huang J, Fu Z et al (2015) The effects of atorvastatin on the prevention of osteoporosis and dyslipidemia in the high-fat-fed ovariectomized rats. Calcif Tissue Int 96:541–551. https://doi.org/10.1007/s00223-015-9975-7
Ko FC, Karim L, Brooks DJ et al (2017) Bisphosphonate withdrawal: effects on bone formation and bone resorption in maturing male mice. J Bone Miner Res 32:814–820. https://doi.org/10.1002/jbmr.3052
Samadfam R, Xia Q, Goltzman D (2007) Co-treatment of PTH with osteoprotegerin or alendronate increases its anabolic effect on the skeleton of oophorectomized mice. J Bone Miner Res 22:55–63. https://doi.org/10.1359/jbmr.060915
Bouxsein ML, Boyd SK, Christiansen BA et al (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486. https://doi.org/10.1002/jbmr.141
Lin S, Huang J, Zheng L et al (2014) Glucocorticoid-Induced Osteoporosis in Growing Rats. Calcif Tissue Int 95:362–373. https://doi.org/10.1007/s00223-014-9899-7
Lin SE, Huang JP, Wu LZ et al (2013) Prevention of osteopenia and dyslipidemia in rats after ovariectomy with combined aspirin and low-dose diethylstilbestrol. Biomed Environ Sci 26:249–257. https://doi.org/10.3967/0895-3988.2013.04.003
Dempster DW, Compston JE, Drezner MK et al (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28:2–17. https://doi.org/10.1002/jbmr.1805
Freudlsperger C, Bian Y, Wise SC et al (2012) TGF- b and NF- k B signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 32:1549–1559. https://doi.org/10.1038/onc.2012.171
Gohil KJ, Patel JA, Gajjar AK (2010) Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci 72:546–556. https://doi.org/10.4103/0250-474X.78519
Wu T, Geng J, Guo W et al (2017) Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharm Sin B 7:65–72. https://doi.org/10.1016/j.apsb.2016.04.003
Yun K, Kim J, Kim J et al (2008) Inhibition of LPS-induced NO and PGE 2 production by asiatic acid via NF-κB inactivation in RAW 264. 7 macrophages: possible involvement of the IKK and MAPK pathways. Int Immunopharmacol 8:431–441. https://doi.org/10.1016/j.intimp.2007.11.003
Maquart FX, Chastang F, Simeon A, Birembaut P, Gillery PWY (1999) Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. Eur J Dermatol 9:289–296
Ramanathan M, Sivakumar S, Anandvijayakumar PR et al (2007) Neuroprotective evaluation of standardized extract of centella asciatica in monosodium glutamate treated rats. Indian J Exp Biol 45:425–431
Tang X-L, Yang X-Y, Jung H-J et al (2009) Asiatic acid induces colon cancer cell growth inhibition and apoptosis through mitochondrial death cascade. Biol Pharm Bull 32:1399–1405. https://doi.org/10.1248/bpb.32.1399
Hao C, Wu B, Hou Z et al (2017) Asiatic acid inhibits LPS-induced inflammatory response in human gingival fibroblasts. Int Immunopharmacol 50:313–318. https://doi.org/10.1016/j.intimp.2017.07.005
Mizukami J, Takaesu G, Akatsuka H et al (2002) Receptor activator of NF-κB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB 2, and TRAF6. Mol Cell Biol 22:992–1000. https://doi.org/10.1128/MCB.22.4.992-1000.2002
Kobayashi Y, Mizoguchi T, Take I et al (2005) Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK1. J Biol Chem 280:11395–11403. https://doi.org/10.1074/jbc.M411189200
Yan X, Liu Z, Chen Y (2009) Regulation of TGF- b signaling by Smad7 overview of TGF-b signaling pathways. Acta Biochim Biophys Sin (Shanghai) 41:263–272. https://doi.org/10.1093/abbs/gmp018.Review
Li X, Ominsky MS, Warmington KS et al (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24:578–588. https://doi.org/10.1359/jbmr.081206
Acknowledgements
This work was supported by grants from Hong Kong Government Research Grant Council, General Research Fund (14119115, 14120118, 14160917, 9054014 N_CityU102/15 and T13-402/17-N), Health and Medical Research Fund (16170951), the National Natural Science Foundation of China (81874000, 81430049, and 81772322), Natural Science Foundation of Guangdong Science and Technology Department (2018A030313374, 2016B030309002), Guangdong Medical Sciences and Technology Fund (A2018111), Guangdong Traditional Chinese Medicine Bureau Fund (20191184), and funds from Affiliated Hospital of Guangdong Medical University (526Z20170001, 21201DFK20190057). This study was also supported in part by SMART program, Lui Che Woo Institute of Innovative Medicine, The Chinese University of Hong Kong and the research was made possible by resources donated by Lui Che Woo Foundation Limited.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Jianping Huang, Haixing Wang, Meiling Huang, Zhixian Zong, Xinyou Wu, Jianbin Xu, Huiyao Lan, Jinchang Zheng, Xiaoting Zhang, Yuk Wai Lee, Bo Wei, Liao Cui, Sien Lin, and Gang Li declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All animal experiments were approved by the Animal Experimental Ethical Committee of the Chinese University of Hong Kong (Ref. No.: 15-081-MIS).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, J., Wang, H., Huang, M. et al. Asiatic Acid Attenuates Bone Loss by Regulating Osteoclastic Differentiation. Calcif Tissue Int 105, 531–545 (2019). https://doi.org/10.1007/s00223-019-00596-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00596-z