Abstract
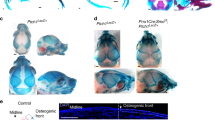
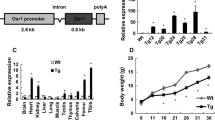
Increasing evidence has demonstrated the important role of autophagy in skeletal homeostasis; however, the role of autophagy in craniofacial bone development and acquisition is largely unknown. In this study, we investigated the effect of autophagy suppression on craniofacial bone acquisition by deleting Fip200 or Atg5, two essential autophagy genes, using Osterix-Cre (Osx-Cre). We found that the Osx-Cre transgene mildly decreased the bone mass of parietal bone but not frontal bone, and did not affect cranial base bone mass in adult mice. In the cranial vault, Fip200 or Atg5 deletion similarly decreased 50% bone mass of neural crest-derived frontal bone; Atg5 deletion decreased 50% and Fip200 deletion decreased 30% bone mass of mesoderm-derived parietal bone. In the cranial base, Fip200 or Atg5 deletion similarly decreased 30% bone mass of neural crest-derived presphenoid bone; Atg5 deletion decreased 30% and Fip200 deletion decreased 16% bone mass of mesoderm-derive basioccipital bone. Lastly, we used doxycycline treatment to inhibit the Osx-Cre expression until 2 months of age and showed that postnatal Fip200 deletion led to cranial vault bone mass decrease in association with a small increase in both bone volume/tissue volume and tissue mineral density. Altogether, this study demonstrated the important role of autophagy in craniofacial bone acquisition during development and postnatal growth.








Similar content being viewed by others
References
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Kundu M, Thompson CB (2008) Autophagy: basic principles and relevance to disease. Annu Rev Pathol 3:427–455
Rabinowitz JD, White E (2010) Autophagy and metabolism. Science 330:1344–1348
Zhang L, Guo YF, Liu YZ, Liu YJ, Xiong DH, Liu XG, Wang L, Yang TL, Lei SF, Guo Y, Yan H, Pei YF, Zhang F, Papasian CJ, Recker RR, Deng HW (2010) Pathway-based genome-wide association analysis identified the importance of regulation-of-autophagy pathway for ultradistal radius BMD. J Bone Miner Res 25:1572–1580
Pan F, Liu XG, Guo YF, Chen Y, Dong SS, Qiu C, Zhang ZX, Zhou Q, Yang TL, Guo Y, Zhu XZ, Deng HW (2010) The regulation-of-autophagy pathway may influence Chinese stature variation: evidence from elder adults. J Hum Genet 55:441–447
DeSelm CJ, Miller BC, Zou W, Beatty WL, van Meel E, Takahata Y, Klumperman J, Tooze SA, Teitelbaum SL, Virgin HW (2011) Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell 21:966–974
Onal M, Piemontese M, Xiong J, Wang Y, Han L, Ye S, Komatsu M, Selig M, Weinstein RS, Zhao H, Jilka RL, Almeida M, Manolagas SC, O’Brien CA (2013) Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem 288:17432–17440
Piemontese M, Onal M, Xiong J, Wang Y, Almeida M, Thostenson JD, Weinstein RS, Manolagas SC, O’Brien CA (2015) Suppression of autophagy in osteocytes does not modify the adverse effects of glucocorticoids on cortical bone. Bone 75:18–26
Kang X, Yang W, Feng D, Jin X, Ma Z, Qian Z, Xie T, Li H, Liu J, Wang R, Li F, Li D, Sun H, Wu S (2017) Cartilage-specific autophagy deficiency promotes ER stress and impairs chondrogenesis in PERK-ATF4-CHOP-dependent manner. J Bone Miner Res 32:2128–2141
Vuppalapati KK, Bouderlique T, Newton PT, Kaminskyy VO, Wehtje H, Ohlsson C, Zhivotovsky B, Chagin AS (2015) Targeted deletion of autophagy genes Atg5 or Atg7 in the chondrocytes promotes caspase-dependent cell death and leads to mild growth retardation. J Bone Miner Res 30:2249–2261
Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L, Goldstein SA, Krebsbach PH, Guan JL (2013) Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res 28:2414–2430
Nollet M, Santucci-Darmanin S, Breuil V, Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S, Cailleteau L, Battaglia S, Farlay D, Dacquin R, Barois N, Jurdic P, Boivin G, Heymann D, Lafont F, Lu SS, Dempster DW, Carle GF, Pierrefite-Carle V (2014) Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 10:1965–1977
Piemontese M, Onal M, Xiong J, Han L, Thostenson JD, Almeida M, O’Brien CA (2016) Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Sci Rep 6:24262
Li H, Li D, Ma Z, Qian Z, Kang X, Jin X, Li F, Wang X, Chen Q, Sun H, Wu S (2018) Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy 14:1726–1741
Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU (2006) Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 8:1124–1132
Hwang S, Maloney NS, Bruinsma MW, Goel G, Duan E, Zhang L, Shrestha B, Diamond MS, Dani A, Sosnovtsev SV, Green KY, Lopez-Otin C, Xavier RJ, Thackray LB, Virgin HW (2012) Nondegradative role of Atg5-Atg12/Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell Host Microbe 11:397–409
Lee IH, Kawai Y, Fergusson MM, Rovira II, Bishop AJ, Motoyama N, Cao L, Finkel T (2012) Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 336:225–228
Han J, Hou W, Goldstein LA, Stolz DB, Watkins SC, Rabinowich H (2014) A complex between Atg7 and Caspase-9: A NOVEL MECHANISM OF CROSS-REGULATION BETWEEN AUTOPHAGY AND APOPTOSIS. J Biol Chem 289:6485–6497
Abbi S, Ueda H, Zheng C, Cooper LA, Zhao J, Christopher R, Guan JL (2002) Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol Biol Cell 13:3178–3191
Ueda H, Abbi S, Zheng C, Guan JL (2000) Suppression of Pyk2 kinase and cellular activities by FIP200. J Cell Biol 149:423–430
Bestebroer J, V’Kovski P, Mauthe M, Reggiori F (2013) Hidden behind autophagy: the unconventional roles of ATG proteins. Traffic 14:1029–1041
Thorburn A (2018) Autophagy and disease. J Biol Chem 293:5425–5430
Subramani S, Malhotra V (2013) Non-autophagic roles of autophagy-related proteins. EMBO Rep 14:143–151
Mizushima N (2018) A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol 20:521–527
Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N (2008) FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol 181:497–510
Hara T, Mizushima N (2009) Role of ULK-FIP200 complex in mammalian autophagy: FIP200, a counterpart of yeast Atg17? Autophagy 5:85–87
Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20:1981–1991
Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20:1992–2003
Ganley IG, du Lam H, Wang J, Ding X, Chen S, Jiang X (2009) ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 284:12297–12305
Chan EY (2009) mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci Signal 2:pe51
Mizushima N (2010) The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 22:132–139
Mizushima N, Ohsumi Y, Yoshimori T (2002) Autophagosome formation in mammalian cells. Cell Struct Funct 27:421–429
Wei X, Thomas N, Hatch NE, Hu M, Liu F (2017) Postnatal craniofacial skeletal development of female C57BL/6NCrl Mice. Front Physiol 8:697
Rodda SJ, McMahon AP (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133:3231–3244
Gan B, Peng X, Nagy T, Alcaraz A, Gu H, Guan JL (2006) Role of FIP200 in cardiac and liver development and its regulation of TNFalpha and TSC-mTOR signaling pathways. J Cell Biol 175:121–133
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889
Liu F, Lee JY, Wei H, Tanabe O, Engel JD, Morrison SJ, Guan JL (2010) FIP200 is required for the cell-autonomous maintenance of fetal hematopoietic stem cells. Blood 116:4806–4814
Wei X, Hu M, Liu F (2018) Mid-facial developmental defects caused by the widely used LacZ reporter gene when expressed in neural crest-derived cells. Transgenic Res 27:551–558
Choi HK, Yuan H, Fang F, Wei X, Liu L, Li Q, Guan JL, Liu F (2018) Tsc1 regulates the balance between osteoblast and adipocyte differentiation through autophagy/Notch1/beta-catenin cascade. J Bone Miner Res 33:2021–2034
Sun C, Yuan H, Wang L, Wei X, Williams L, Krebsbach PH, Guan JL, Liu F (2016) FAK promotes osteoblast progenitor cell proliferation and differentiation by enhancing Wnt signaling. J Bone Miner Res 31:2227–2238
Fang F, Sun S, Wang L, Guan JL, Giovannini M, Zhu Y, Liu F (2015) Neural crest-specific TSC1 deletion in mice leads to sclerotic craniofacial bone lesion. J Bone Miner Res 30:1195–1205
Wang L, Mishina Y, Liu F (2015) Osterix-Cre transgene causes craniofacial bone development defect. Calcif Tissue Int 96:129–137
Gan B, Guan JL (2008) FIP200, a key signaling node to coordinately regulate various cellular processes. Cell Signal 20:787–794
Quarto N, Wan DC, Kwan MD, Panetta NJ, Li S, Longaker MT (2010) Origin matters: differences in embryonic tissue origin and Wnt signaling determine the osteogenic potential and healing capacity of frontal and parietal calvarial bones. J Bone Miner Res 25:1680–1694
Hoshi K, Ozawa H (2000) Matrix vesicle calcification in bones of adult rats. Calcif Tissue Int 66:430–434
Rohde M, Mayer H (2007) Exocytotic process as a novel model for mineralization by osteoblasts in vitro and in vivo determined by electron microscopic analysis. Calcif Tissue Int 80:323–336
Dunlop LL, Hall BK (1995) Relationships between cellular condensation, preosteoblast formation and epithelial-mesenchymal interactions in initiation of osteogenesis. Int J Dev Biol 39:357–371
Hall BK, Miyake T (2000) All for one and one for all: condensations and the initiation of skeletal development. BioEssays 22:138–147
Davey RA, Clarke MV, Sastra S, Skinner JP, Chiang C, Anderson PH, Zajac JD (2012) Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic Res 21:885–893
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241
Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J (2012) Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res 27:2344–2358
Hu K, Olsen BR (2016) Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Investig 126:509–526
Acknowledgements
We thank Dr. Noboru Mizushima for providing the Atg5 floxed mice. This study was funded by the NIH (Grant Nos. AR062030 to F.L, NS094144 to JG) and University of Michigan School of Dentistry Research Pathway fund (to N.T). MicroCT work was partly supported by P30 Core Center award to University of Michigan from NIAMS (AR 69620).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Neil Thomas, Han Kyoung Choi, Xiaoxi Wei, Li Wang, Yuji Mishina, Jun-Lin Guan, and Fei Liu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human subjects performed by any of the authors. All mice procedures used in this study were approved by the Institutional Animal Care and Use Committee at the University of Michigan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thomas, N., Choi, H.K., Wei, X. et al. Autophagy Regulates Craniofacial Bone Acquisition. Calcif Tissue Int 105, 518–530 (2019). https://doi.org/10.1007/s00223-019-00593-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00593-2