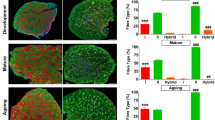
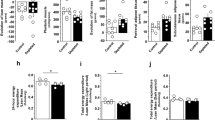
Abstract
This special issue article will focus on morphologic and functional roles of vitamin D in muscle, from strength to contraction to development and ageing and will characterise the controversy of VDR’s expression in skeletal muscle, central to our understanding of vitamin D’s effects on this tissue.
Similar content being viewed by others
References
Floyd M, Ayyar DR, Barwick DD, Hudgson P, Weightman D (1974) Myopathy in chronic renal failure. Q J Med 43(172):509–524
Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE (2013) The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev 34(1):33–83
Whistler D (1645) De morbo puerili Anglorum quem patrio idiomate indigenae vocant. The Rickets. Londini, London
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96(1):53–58
Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF et al (2008) Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29(6):726–776
Cui X, Pelekanos M, Liu PY, Burne TH, McGrath JJ, Eyles DW (2013) The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience 236:77–87
Brumbaugh PF, Haussler MR (1974) 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. I. Association of 1 alpha,25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem 249(4):1251–1257
Girgis CM (2018) Vitamin D and skeletal muscle. In: Feldman DJPW, Bouillon R, Giovanucci E, Goltzman D, Hewison M (eds) Vitamin D. Elsevier, Atlanta, pp 597–613
Wang Y, Becklund BR, DeLuca HF (2010) Identification of a highly specific and versatile vitamin D receptor antibody. Arch Biochem Biophys 494(2):166–177
Simpson RU, Thomas GA, Arnold AJ (1985) Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem 260(15):8882–8891
Costa EM, Blau HM, Feldman D (1986) 1,25-Dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology 119(5):2214–2220
Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T et al (2003) Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 144(12):5138–5144
Ceglia L, da Silva Morais M, Park LK, Morris E, Harris SS, Bischoff-Ferrari HA et al (2010) Multi-step immunofluorescent analysis of vitamin D receptor loci and myosin heavy chain isoforms in human skeletal muscle. J Mol Histol 41(2–3):137–142
Girgis CM, Mokbel N, Minn Cha K, Houweling PJ, Abboud M, Fraser DR et al (2014) The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 155(9):3227–3237
Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB et al (2001) In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 33(1):19–24
Buitrago C, Boland R (2010) Caveolae and caveolin-1 are implicated in 1alpha,25(OH)2-vitamin D3-dependent modulation of Src, MAPK cascades and VDR localization in skeletal muscle cells. J Steroid Biochem Mol Biol 121(1–2):169–175
Wang Y, DeLuca HF (2011) Is the vitamin d receptor found in muscle? Endocrinology 152(2):354–363
Olsson K, Saini A, Stromberg A, Alam S, Lilja M, Rullman E et al (2016) Evidence for vitamin D receptor expression and direct effects of 1 alpha, 25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology 157(1):98–111
Ceglia L, Niramitmahapanya S, Morais MD, Rivas DA, Harris SS, Bischoff-Ferrari H et al (2013) A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab 98(12):1927–1935
Pojednic RM, Ceglia L, Olsson K, Gustafsson T, Lichtenstein AH, Dawson-Hughes B et al (2015) Effects of 1,25-dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin d receptor in human skeletal muscle cells. Calcif Tissue Int 96(3):256–263
Lee SM, Bishop KA, Goellner JJ, O’Brien CA, Pike JW (2014) Mouse and human BAC transgenes recapitulate tissue-specific expression of the vitamin D receptor in mice and rescue the VDR-null phenotype. Endocrinology 155(6):2064–2076
Capiati D, Benassati S, Boland RL (2002) 1,25(OH)2-Vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem 86(1):128–135
Buitrago C, de Boland R, Boland AR (2001) The tyrosine kinase c-Src is required for 1,25(OH)2-vitamin D3 signalling to the nucleus in muscle cells. Biochimica et Biophysica Acta. 1541(3):179–187
Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN (2011) 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 152(8):2976–2986
Makanae Y, Ogasawara R, Sato K, Takamura Y, Matsutani K, Kido K et al (2015) Acute bout of resistance exercise increases vitamin D receptor protein expression in rat skeletal muscle. Exp Physiol 100(10):1168–1176
Srikuea R, Zhang X, Park-Sarge OK, Esser KA (2012) VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol 303(4):C396–C405
Cheng C, Alexander R, Min R, Leng J, Yip KY, Rozowsky J et al (2012) Understanding transcriptional regulation by integrative analysis of transcription factor binding data. Genome Res 22(9):1658–1667
Yin H, Price F, Rudnicki MA (2013) Satellite cells and the muscle stem cell niche. Physiol Rev 93(1):23–67
Johnson JA, Grande JP, Roche PC, Kumar R (1996) Ontogeny of the 1,25-dihydroxyvitamin D3 receptor in fetal rat bone. J Bone Miner Res 11(1):56–61
Artaza JN, Norris KC (2009) Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol 200(2):207–221
Garcia LA, Ferrini MG, Norris KC, Artaza JN (2013) 1,25(OH)(2)Vitamin D(3) enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C(2)C(12) skeletal muscle cells. J Steroid Biochem Mol Biol 133:1–11
Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Gunton JE (2014) Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 155(2):347–357
Morelli S, Buitrago C, Boland R, de Boland AR (2001) The stimulation of MAP kinase by 1,25(OH)(2)-vitamin D(3) in skeletal muscle cells is mediated by protein kinase C and calcium. Mol Cell Endocrinol 173(1–2):41–52
Buitrago CG, Pardo VG, de Boland AR, Boland R (2003) Activation of RAF-1 through Ras and protein kinase Calpha mediates 1alpha,25(OH)2-vitamin D3 regulation of the mitogen-activated protein kinase pathway in muscle cells. J Biol Chem 278(4):2199–2205
Ronda AC, Buitrago C, Colicheo A, de Boland AR, Roldan E, Boland R (2007) Activation of MAPKs by 1alpha,25(OH)2-Vitamin D3 and 17beta-estradiol in skeletal muscle cells leads to phosphorylation of Elk-1 and CREB transcription factors. J Steroid Biochem Mol Biol 103(3–5):462–466
Boland R, De Boland AR, Buitrago C, Morelli S, Santillan G, Vazquez G et al (2002) Non-genomic stimulation of tyrosine phosphorylation cascades by 1,25(OH)(2)D(3) by VDR-dependent and independent mechanisms in muscle cells. Steroids 67(6):477–482
Tanaka M, Kishimoto KN, Okuno H, Saito H, Itoi E (2014) Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve 49(5):700–708
Girgis CM, Cha KM, Houweling PJ, Rao R, Mokbel N, Lin M et al (2015) Vitamin D receptor ablation and vitamin D deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcif Tissue Int 97(6):602–610
Seoane S, Alonso M, Segura C, Perez-Fernandez R (2002) Localization of a negative vitamin D response sequence in the human growth hormone gene. Biochem Biophys Res Commun 292(1):250–255
Sakoda K, Fujiwara M, Arai S, Suzuki A, Nishikawa J, Imagawa M et al (1996) Isolation of a genomic DNA fragment having negative vitamin D response element. Biochem Biophys Res Commun 219(1):31–35
Chen S, Villalta SA, Agrawal DK (2016) FOXO1 mediates vitamin D deficiency-induced insulin resistance in skeletal muscle. J Bone Miner Res 31(3):585–595
Krishnaveni GV, Veena SR, Winder NR, Hill JC, Noonan K, Boucher BJ et al (2011) Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: the Mysore Parthenon Study. Am J Clin Nutr 93(3):628–635
Harvey NC, Moon RJ, Sayer AA, Ntani G, Davies JH, Javaid MK et al (2014) Maternal antenatal vitamin D status and offspring muscle development: findings from the Southampton Women’s Survey. J Clin Endocrinol Metab 99(1):330–337
Max D, Brandsch C, Schumann S, Kuhne H, Frommhagen M, Schutkowski A et al (2013) Maternal vitamin D deficiency causes smaller muscle fibers and altered transcript levels of genes involved in protein degradation, myogenesis, and cytoskeleton organization in the newborn rat. Mol Nutr Food Res 58:343–352
Oku Y, Tanabe R, Nakaoka K, Yamada A, Noda S, Hoshino A et al (2016) Influences of dietary vitamin D restriction on bone strength, body composition and muscle in rats fed a high-fat diet: involvement of mRNA expression of MyoD in skeletal muscle. J Nutr Biochem 32:85–90
Zhou H, Chen Y, Lv G, Zhuo Y, Lin Y, Feng B et al (2016) Improving maternal vitamin D status promotes prenatal and postnatal skeletal muscle development of pig offspring. Nutrition 32(10):1144–1152
Alami-Durante H, Cluzeaud M, Bazin D, Mazurais D, Zambonino-Infante JL (2011) Dietary cholecalciferol regulates the recruitment and growth of skeletal muscle fibers and the expressions of myogenic regulatory factors and the myosin heavy chain in European sea bass larvae. J Nutr 141(12):2146–2151
Harvey NC, Sheppard A, Godfrey KM, McLean C, Garratt E, Ntani G et al (2014) Childhood bone mineral content is associated with methylation status of the RXRA promoter at birth. J Bone Miner Res 29(3):600–607
Girgis CM, Clifton-Bligh RJ, Turner N, Lau SL, Gunton JE (2014) Effects of vitamin D in skeletal muscle: falls, strength, athletic performance and insulin sensitivity. Clin Endocrinol (Oxf). 80(2):169–181
Burne TH, Johnston AN, McGrath JJ, Mackay-Sim A (2006) Swimming behaviour and post-swimming activity in Vitamin D receptor knockout mice. Brain Res Bull 69(1):74–78
Minasyan A, Keisala T, Zou J, Zhang Y, Toppila E, Syvala H et al (2009) Vestibular dysfunction in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol 114(3–5):161–166
Schubert L, DeLuca HF (2010) Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch Biochem Biophys 500(2):157–161
Rodman JS, Baker T (1978) Changes in the kinetics of muscle contraction in vitamin D-depleted rats. Kidney Int 13(3):189–193
Pleasure D, Wyszynski B, Sumner A, Schotland D, Feldman B, Nugent N et al (1979) Skeletal muscle calcium metabolism and contractile force in vitamin D-deficient chicks. J Clin Investig 64(5):1157–1167
Pointon JJ, Francis MJ, Smith R (1979) Effect of vitamin D deficiency on sarcoplasmic reticulum function and troponin C concentration of rabbit skeletal muscle. Clin Sci (Lond). 57(3):257–263
Stroder J, Arensmeyer E (1965) Actomyosin content of the skeletal muscles in experimental rickets. Klin Wochenschr 43(22):1201–1202
de Boland AR, Albornoz LE, Boland R (1983) The effect of cholecalciferol in vivo on proteins and lipids of skeletal muscle from rachitic chicks. Calcif Tissue Int 35(6):798–805
Curry OB, Basten JF, Francis MJ, Smith R (1974) Calcium uptake by sarcoplasmic reticulum of muscle from vitamin D-deficient rabbits. Nature 249(452):83–84
Tague SE, Clarke GL, Winter MK, McCarson KE, Wright DE, Smith PG (2011) Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyperinnervation. J Neurosci 31(39):13728–13738
Sakai S, Suzuki M, Tashiro Y, Tanaka K, Takeda S, Aizawa K et al (2015) Vitamin D receptor signaling enhances locomotive ability in mice. J Bone Miner Res 30(1):128–136
Abboud M, Puglisi DA, Davies BN, Rybchyn M, Whitehead NP, Brock KE et al (2013) Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology 154(9):3022–3030
Vazquez G, Boland R, de Boland AR (1995) Modulation by 1,25(OH)2-vitamin D3 of the adenylyl cyclase/cyclic AMP pathway in rat and chick myoblasts. Biochim Biophys Acta 1269(1):91–97
Capiati DA, Vazquez G, Boland RL (2001) Protein kinase C alpha modulates the Ca2+ influx phase of the Ca2+ response to 1alpha,25-dihydroxy-vitamin-D3 in skeletal muscle cells. Horm Metab Res 33(4):201–206
Boland RL (2011) VDR activation of intracellular signaling pathways in skeletal muscle. Mol Cell Endocrinol 347(1–2):11–16
Morelli S, de Boland AR, Boland RL (1993) Generation of inositol phosphates, diacylglycerol and calcium fluxes in myoblasts treated with 1,25-dihydroxyvitamin D3. Biochem J. 289(Pt 3):675–679
Berchtold MW, Brinkmeier H, Muntener M (2000) Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 80(3):1215–1265
Sohl E, van Schoor NM, de Jongh RT, Visser M, Deeg DJ, Lips P (2013) Vitamin d status is associated with functional limitations and functional decline in older individuals. J Clin Endocr Metab 98(9):E1483–E1490
Bhat M, Kalam R, Qadri SS, Madabushi S, Ismail A (2013) Vitamin D deficiency induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology 154(11):4018–4029
Antinozzi C, Corinaldesi C, Giordano C, Pisano A, Cerbelli B, Migliaccio S et al (2017) Potential role for the VDR agonist elocalcitol in metabolic control: evidences in human skeletal muscle cells. J Steroid Biochem Mol Biol 167:169–181
Montero-Odasso M, Duque G (2005) Vitamin D in the aging musculoskeletal system: an authentic strength preserving hormone. Mol Aspects Med 26(3):203–219
Phelps M, Pettan-Brewer C, Ladiges W, Yablonka-Reuveni Z (2013) Decline in muscle strength and running endurance in klotho deficient C57BL/6 mice. Biogerontology 14(6):729–739
Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C et al (2012) Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur J Appl Physiol 112(4):1215–1220
Bhat M, Ismail A (2015) Vitamin D treatment protects against and reverses oxidative stress induced muscle proteolysis. J Steroid Biochem Mol Biol 152:171–179
Sinha A, Hollingsworth KG, Ball S, Cheetham T (2013) Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocr Metab 98(3):E509–E513
Al-Eisa ES, Alghadir AH, Gabr SA (2016) Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin Interv Aging 11:513–522
Ryan ZC, Craig TA, Folmes CD, Wang X, Lanza IR, Schaible NS et al (2016) 1alpha,25-Dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J Biol Chem 291(3):1514–1528
Owens DJ, Sharples AP, Polydorou I, Alwan N, Donovan T, Tang J et al (2015) A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am J Physiol Endocrinol Metab. 309(12):E1019–E1031
Stratos I, Li Z, Herlyn P, Rotter R, Behrendt AK, Mittlmeier T et al (2013) Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Am J Pathol 182(3):895–904
Choi M, Park H, Cho S, Lee M (2013) Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high-intensity exercise in SD rats. Cytokine 63(1):27–35
Ke CY, Yang FL, Wu WT, Chung CH, Lee RP, Yang WT et al (2016) Vitamin D3 reduces tissue damage and oxidative stress caused by exhaustive exercise. Int J Med Sci 13(2):147–153
Srikuea R, Hirunsai M (1985) Effects of intramuscular administration of 1alpha, 25(OH)2D3 during skeletal muscle regeneration on regenerative capacity, muscular fibrosis, and angiogenesis. J Appl Physiol 120(12):1381–1393
Barker T, Henriksen VT, Martins TB, Hill HR, Kjeldsberg CR, Schneider ED et al (2013) Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients 5(4):1253–1275
Barker T, Martins TB, Hill HR, Kjeldsberg CR, Dixon BM, Schneider ED et al (2014) Vitamin D sufficiency associates with an increase in anti-inflammatory cytokines after intense exercise in humans. Cytokine 65(2):134–137
Pojednic RM, Ceglia L, Lichtenstein AH, Dawson-Hughes B, Fielding RA (2015) Vitamin D receptor protein is associated with interleukin-6 in human skeletal muscle. Endocrine 49(2):512–520
Barker T, Schneider ED, Dixon BM, Henriksen VT, Weaver LK (2013) Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise. Nutr Metab (Lond). 10(1):69
Buitrago C, Vazquez G, De Boland AR, Boland RL (2000) Activation of Src kinase in skeletal muscle cells by 1, 1,25-(OH(2))-vitamin D(3) correlates with tyrosine phosphorylation of the vitamin D receptor (VDR) and VDR-Src interaction. J Cell Biochem 79(2):274–281
Sandgren ME, Bronnegard M, DeLuca HF (1991) Tissue distribution of the 1,25-dihydroxyvitamin D3 receptor in the male rat. Biochem Biophys Res Commun 181(2):611–616
Roh YH, Hong SW, Chung SW, Lee YS (2019) Altered gene and protein expressions of vitamin D receptor in skeletal muscle in sarcopenic patients who sustained distal radius fractures. J Bone Miner Metab. https://doi.org/10.1007/s00774-019-00995-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Christian M. Girgis declares that he has no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Girgis, C.M. Vitamin D and Skeletal Muscle: Emerging Roles in Development, Anabolism and Repair. Calcif Tissue Int 106, 47–57 (2020). https://doi.org/10.1007/s00223-019-00583-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00583-4