Abstract
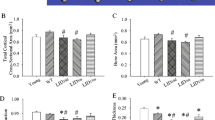
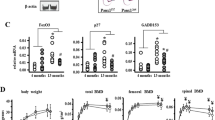
Peroxisome proliferator-activated receptor-gamma coactivator (PGC1α) is a transcription coactivator that interacts with a broad range of transcription factors involved in several biological responses. Here, we show that PGC1α plays a role in skeletal homeostasis since aged PGC1α-deficient mice (PGC1α−/−) display impaired bone structure. Micro-CT of the tibial mid-shaft showed a marked decrease of cortical thickness in PGC1α−/− (− 11.9%, p < 0.05) mice compared to wild-type littermate. Trabecular bone was also impaired in knock out mice which displayed lower trabecular thickness (Tb.Th) (− 5.9% vs PGC1α+/+, p < 0.05), whereas trabecular number (Tb.N) was higher than wild-type mice (+ 72% vs PGC1α+/+, p < 0.05), thus resulting in increased (+ 31.7% vs PGC1α+/+, p < 0.05) degree of anisotropy (DA), despite unchanged bone volume fraction (BV/TV). Notably, these impairments of cortical and trabecular bone led to a dramatic ~ 48.4% decrease in bending strength (p < 0.01). These changes in PGC1α−/− mice were paralleled by a significant increase in osteoclast number at the cortical bone surface and in serum level of the bone resorption marker, namely, C-terminal cross-linked telopeptides of type I collagen (CTX-I). We also found that in cortical bone, there was lower expression of mRNA codifying for the key bone-building protein Osteocalcin (Ocn). Interestingly, Collagen I mRNA expression was reduced in mesenchymal stem cells from bone marrow of PGC1α−/−, thus indicating that differentiation of osteoblast lineage is downregulated. Overall, results presented herein suggest that PGC1α may play a key role in bone metabolism.






Similar content being viewed by others
References
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418(6899):797–801
Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM (2001) Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98(7):3820–3825
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92(6):829–839
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98(1):115–124
Lin J, Tarr PT, Yang R, Rhee J, Puigserver P, Newgard CB, Spiegelman BM (2003) PGC-1β in the regulation of hepatic glucose and energy metabolism. J Biol Chem 278(33):30843–30848
Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA (2003) PPARγ coactivator-1α expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol 284(6):C1669–C1677
Ljubicic V, Joseph AM, Saleem A, Uguccioni G, Collu-Marchese M, Lai RY, Nguyen LM, Hood DA (2010) Transcriptional and post-transcriptional regulation of mitochondrial biogenesis in skeletal muscle: effects of exercise and aging. Biochem Biophys Acta 1800(3):223–234
Pilegaard H, Saltin B, Neufer PD (2003) Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546(3):851–858
Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO (2002) Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. Fed Am Soc Exp Biol 16(14):1879–1886
Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T (2000) cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 274(2):350–354
Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM (2001) Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413(6852):131–138
Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM (2004) Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119(1):121–135
Nervina JM, Magyar CE, Pirih FQ, Tetradis S (2006) PGC-1alpha is induced by parathyroid hormone and coactivates Nurr1-mediated promoter activity in osteoblasts. Bone 39(5):1018–1025
D’Errico I, Salvatore L, Murzilli S, Lo Sasso G, Latorre D, Martelli N, Egorova AV, Polishuck R, Madeyski-Bengtson K, Lelliott C, Vidal-Puig AJ, Seibel P, Villani G, Moschetta A (2011) Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) is a metabolic regulator of intestinal epithelial cell fate. Proc Natl Acad Sci USA 108(16):6603–6608
Uguccioni G, Hood DA (2011) The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am J Physiol Endocrinol Metab 300(2):E361–E371
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481(7382):463–468
Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Soc 24:78–90
Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM (2003) An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci USA 100:7111–7116
Handschin C, Spiegelman BM (2011) PGC-1 coactivators and the regulation of skeletal muscle fiber-type determination. [Comment Lett] Cell Metab 13(4):351 (author reply 352).
Brotto M, Johnson ML (2014) Endocrine crosstalk between muscle and bone. Curr Osteoporo Rep 12(2):135–141
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, New MI, Zaidi M, Cinti S, Grano M (2015) The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA 112(39):12157–12162
Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galán-Díez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, Bacchetta J, Szulc P, Kitsis RN, de Cabo R, Friedman RA, Torsitano C, McGraw TE, Puchowicz M, Kurland I, Karsenty G (2016) Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab 23(6):1078–1092
Kersh ME, Zysset PK, Pahr DH, Wolfram U, Larsson D, Pandy MG (2013) Measurement of structural anisotropy in femoral trabecular bone using clinical-resolution CT images. J Biomech 46(15):2659–2666
Chappard C, Brunet-Imbault B, Lemineur G, Giraudeau B, Basillais A, Harba R, Benhamou CL (2005) Anisotropy changes in post-menopausal osteoporosis: characterization by a new index applied to trabecular bone radiographic images. Osteoporos Int 16(10):1193–1202
Singh M, Nagrath AR, Maini PS (1970) Changes in trabecular pattern of the upper end of the femur as an index of osteoporosis. J Bone Joint Surg Am 52(3):457–467
Newitt DC, van Rietbergen B, Majumdar S (2002) Processing and analysis of in vivo high-resolution MR images of trabecular bone for longitudinal studies: reproducibility of structural measures and micro-finite element analysis derived mechanical properties. Osteoporos Int 13(4):278–287
Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, McDonnell DP, Unterman TG, Elam MB, Park EA (2006) Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem 281:39897–39906
Wang H, Wang J (2013) Estrogen-related receptor alpha interacts cooperatively with peroxisome proliferator-activated receptor-gamma coactivator-1alpha to regulate osteocalcin gene expression. Cell Biol Int 37(11):1259–1265
Florencio-Silva R, Rodrigues da Silva Sasso G, Sasso-Cerri E, Simões MJ, Cerri PS (2015) Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res Int. https://doi.org/10.1155/2015/421746
Colaianni G, Brunetti G, Faienza MF, Colucci S, Grano M (2014) Osteoporosis and obesity: role of Wnt pathway in human and murine models. World J Orthop 5(3):242–246
Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A (2013) Marrow fat and bone-new perspectives. J Clin Endocrinol Metab 98(3):935–945
Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML (2010) Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res 25:2078–2088
Bredella MA, Fazeli PK, Miller KK, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A (2009) Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab 94(6):2129–2136
Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J (2012) Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab 302(1):E19–E31
Karsenty G, Oury F (2012) Biology without walls: the novel endocrinology of bone. Annu Rev Physiol 74:87–105
Isaia GC, D’Amelio P, Di Bella S, Tamone C (2005) Is leptin the link between fat and bone mass? J Endocrinol Investig 28(10 Suppl):61–65
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130(3):456–469
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111(3):305–317
Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, Clarke CJ, Hannun YA, DePinho RA, Guo XE, Mann JJ, Karsenty G (2013) Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab 17(6):901–915
Rowe GC, Arany Z (2014) Genetic models of PGC-1 and glucose metabolism and homeostasis. Rev Endocr Metab Disord 15(1):21–29
Rosen CJ, Bouxsein ML (2006) Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35–43
Acknowledgements
We thank Dr. Antonio Moschetta (Department of Interdisciplinary Medicine, “Aldo Moro” University of Bari, 70124 Bari, Italy) for the generous gift of PGC1α heterozygous mice to generate the colony. This work was supported in part by MIUR Grant ex60% (to M.G.), by SIOMMMS Grant (to G.C.) and by ERISTO (ESA) Grant (to M.G.).
Author information
Authors and Affiliations
Contributions
GC, LL, UT, SC, MG designed research; LL, LS, MC, NC performed research; GC, LL, GB, GP, JR, ES, MFF, UT, SC, MG analyzed, interpreted and discussed the data; and GC, LL, MG wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
Graziana Colaianni, Luciana Lippo, Lorenzo Sanesi, Giacomina Brunetti, Monica Celi, Nunzio Cirulli, Giovanni Passeri, Janne Reseland, Ernestina Schipani, Maria Felicia Faienza, Umberto Tarantino, Silvia Colucci, and Maria Grano declare that there is no conflict of interest regarding the publication of this paper.
Human and Animal Rights and Informed Consent
This study is in accordance with the European Law Implementation of Directive 2010/63/EU and all experimental protocols were reviewed and approved by the Veterinary Department of the Italian Ministry of Health (Project 522-2016PR). Experimental procedures have been carried out following the standard biosecurity and the institutional safety procedures. For this type of study formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
223_2018_459_MOESM1_ESM.docx
Supplementary Table 1: Effect of PGC1α whole body deletion on cortical and trabecular bone of 3-months old mice. MicroCT analysis of tibia and femurs harvested from 3 months old PGC1α+/+ and PGC1α-/- male (♂) and female (♀) mice. Cortical bone parameters included cortical thickness (Ct.Th), polar moment of inertia (pMOI) and bone mineral density (BMD). Trabecular bone parameters included bone volume/total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), degree of Anisotropy (DA), connectivity density (Conn. Density) and bone mineral density (BMD). Data are presented as mean ± SEM. n = 3–4 mice per group. *p < 0.05 versus PGC1α+/+ (DOCX 16 KB)
223_2018_459_MOESM2_ESM.docx
Supplementary Table 2: Sequence, accession number (NM_) and product length for each primer. Primer sequences, accession number (NM_) and the product length for each primer. All primers span an exon-exon junction. Three housekeeping genes (Glyceraldehyde 3-phosphate dehydrogenase, β2-microglobulin and β-actin) were chosen because they are normally stably expressed in bone and adipose tissues (DOCX 16 KB)
Rights and permissions
About this article
Cite this article
Colaianni, G., Lippo, L., Sanesi, L. et al. Deletion of the Transcription Factor PGC-1α in Mice Negatively Regulates Bone Mass. Calcif Tissue Int 103, 638–652 (2018). https://doi.org/10.1007/s00223-018-0459-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0459-4