Abstract
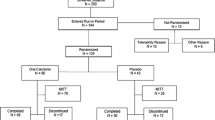
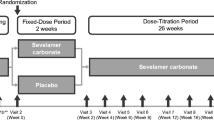
Previous work has demonstrated that a single subcutaneous dose of salmon calcitonin leads to a transient decline in circulating levels of FGF23 in patients with X-linked hypophosphatemia (XLH). Since the calcitonin receptor is expressed on osteocytes, this raises the possibility that interdicting signals through that receptor could modulate circulating levels of FGF23 in XLH. In the present study, 21 subjects with XLH were randomly assigned to receive either placebo nasal spray or 400 IU of nasal salmon calcitonin daily for three months. On the first and last day of the study, serial measurements of FGF23, 1,25-dihydroxyvitamin D, and TmP/GFR were made over 27 h. At the beginning of Visit 2 (the first day of month 2) and the beginning of Visit 3 (the first day of month 3), single, first-morning, fasting measurements of these same parameters were made before the next administered dose of study drug. Following the initial or final dose of study drug, there were no differences in area under the curve, based on treatment assignment, for the three principal outcome variables. Similarly, there were no differences in the fasting measures taken at the beginning of Visit 2 or Visit 3 compared to the fasting values on either day 2 of Visit 1 or the fasting values on day 2 of Visit 4. There were also no significant changes over time in serum phosphorus, serum calcium, circulating levels of PTH, CTx, or P1NP. The reasons why nasal salmon calcitonin did not recapitulate the findings with subcutaneously administered drug may relate to the kinetics of drug delivery, the bioavailability of drug or peak drug dose achieved. It remains possible, however, that other means of altering calcitonin receptor signaling may still provide an opportunity for regulating FGF23 production.

Similar content being viewed by others
References
Goldsweig BK, Carpenter TO (2015) Hypophosphatemic rickets: lessons from disrupted FGF23 control of phosphorus homeostasis. Curr Osteoporos Rep 13:88–97
Francis F, Hennig S, Korn B, Reinhardt R, De Jong P, Poustka A, Lehrach H, Rowe PS, Goulding JN, Summerfield T, Mountford R (1995) A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 11:130–136
Connor J, Olear EA, Insogna KL, Katz L, Baker S, Kaur R, Simpson CA, Sterpka J, Dubrow R, Zhang JH, Carpenter TO (2015) Conventional therapy in adults with X-linked hypophosphatemia: effects on enthesopathy and dental disease. J Clin Endocrinol Metab 100:3625–3632
Carpenter TO, Insogna KL, Zhang JH, Ellis B, Nieman S, Simpson C, Olear E, Gundberg CM (2010) Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab 95:E352–E357
Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL (2011) A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res 26:1381–1388
Carpenter TO, Imel EA, Ruppe MD, Weber TJ, Klausner MA, Wooddell MM, Kawakami T, Ito T, Zhang X, Humphrey J, Insogna KL, Peacock M (2014) Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Investig 124:1587–1597
Imel EA, Zhang X, Ruppe MD, Weber TJ, Klausner MA, Ito T, Vergeire M, Humphrey JS, Glorieux FH, Portale AA, Insogna K, Peacock M, Carpenter TO (2015) Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab 100:2565–2573
Liu ES, Carpenter TO, Gundberg CM, Simpson CA, Insogna KL (2011) Calcitonin administration in X-linked hypophosphatemia. N Engl J Med 364:1678–1680
Kerstetter JE, Caseria DM, Mitnick ME, Ellison AF, Gay LF, Liskov TA, Carpenter TO, Insogna KL (1997) Increased circulating concentrations of parathyroid hormone in healthy, young women consuming a protein-restricted diet. Am J Clin Nutr 66:1188–1196
Kerstetter JE, Bihuniak JD, Brindisi J, Sullivan RR, Mangano KM, Larocque S, Kotler BM, Simpson CA, Cusano AM, Gaffney-Stomberg E, Kleppinger A, Reynolds J, Dziura J, Kenny AM, Insogna KL (2015) The effect of a whey protein supplement on bone mass in older caucasian adults. J Clin Endocrinol Metab 100:2214–2222
Simpson C, Maria Cusano A, Bihuniak J, Walker J, Insogna K (2014) Effect of 25(OH) vitamin D reference method procedure (RMP) alignment on clinical measurements obtained with the IDS-iSYS chemiluminescent-based automated analyzer. J Steroid Biochem Mol Biol 148:41–46
Bijvoet O (1977) Kidney function in calcium and phosphate metabolism. In: Avioli LV, Krane SM (eds) Metabolic bone disease. Academic Press, New York, p 48–140
Overgaard K, Agnusdei D, Hansen MA, Maioli E, Christiansen C, Gennari C (1991) Dose-response bioactivity and bioavailability of salmon calcitonin in premenopausal and postmenopausal women. J Clin Endocrinol Metab 72:344–349
Torring O, Bucht E, Sjostedt U, Sjoberg HE (1991) Salmon calcitonin treatment by nasal spray in primary hyperparathyroidism. Bone 12:311–316
Chesnut CH, Silverman S, Andriano K, Genant H, Gimona A, Harris S, Kiel D, LeBoff M, Maricic M, Miller P, Moniz C, Peacock M, Richardson P, Watts N, Baylink D (2000) A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. Am J Med 109:267–276
Gooi J, Pompolo S, Karsdal M, Kulkarni N, Kalajzic I, McAhren S, Han B, Onyia J, Ho P, Gillespie M, Walsh N, Chia L, Quinn J, Martin T, Sims N (2010) Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone 46:1486–1497
Acknowledgements
The intact FGF23 assay kits were a generous gift from Kyowa Hakko Kirin Co. Ltd. This work was supported by the Yale Bone Center. It was also supported by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Author information
Authors and Affiliations
Contributions
Study design: KI and AA. Data collection: KI, TC, AA, CS, RS, EO. Data analysis: YD, CC, RS, KI, AA, CS. Data interpretation: KI, RS, YD and CC. Drafting manuscript: RS, KI. Approving final version of manuscript: RS, KI, TC, AA, CS, YD, and CC. KI assumes responsibility for the integrity of the data analysis.
Corresponding author
Ethics declarations
Conflict of Interest
Rebecca Sullivan, Alice Abraham, Christine Simpson, Elizabeth Olear, Thomas Carpenter, Yanhong Deng, Chuqing Chen, Karl L. Insogna declare that they have no conflicts of interest relevant to this study.
Human and Animal Rights and Informed Consent
This study was approved by the Yale Human Research Protection Program. All study participants provided written informed consent before study entry.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sullivan, R., Abraham, A., Simpson, C. et al. Three-Month Randomized Clinical Trial of Nasal Calcitonin in Adults with X-linked Hypophosphatemia. Calcif Tissue Int 102, 666–670 (2018). https://doi.org/10.1007/s00223-017-0382-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0382-0