Abstract
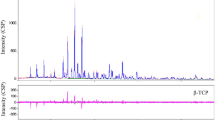
Sinus lift elevation restores bone mass at the maxilla in edentulate patients before the placement of dental implants. It consists of opening the lateral side of the sinus and grafting beta-tricalcium phosphate granules (β-TCP) under the olfactory membrane. Bone biopsies were obtained in five patients after 60 weeks. They were embedded undecalcified in poly(methyl methacrylate) (pMMA); blocks were analyzed by nanocomputed tomography (nanoCT); specific areas were studied by Raman microspectroscopy. Remnants of β-TCP were osseointegrated and covered with mineralized bone; osteoid tissue was also filling the inner porosity. Macrophages having engulfed numerous β-TCP grains were observed in marrow spaces. β-TCP was identified by nanoCT as osseointegrated particles and as granules in the cytoplasm of macrophages. Raman microspectroscopy permitted to compare the spectra of β-TCP and bone in different areas. The ratio of the ~820 cm−1 band of pMMA (–CH2 groups) on the ν1 phosphate band at 960 cm−1 reflected tissue hydration because water was substituted by MMA during histological processing. In bone, the ratio of the ~960 cm−1 phosphate to the amide 1 band and the ratio ν2 phosphate band by the 1240–1250 amide III band reflect the mineralization degree. Specific bands of β-TCP were found in osseointegrated β-TCP granules and in the grains phagocytized by the macrophages. The hydration degree was maximal for β-TCP phagocytized by macrophages. Raman microspectroscopy associated with nanoCT is a powerful tool in the analysis of the biomaterial degradation and osseointegration.






Similar content being viewed by others
References
Bohner M (2010) Resorbable biomaterials as bone graft substitutes. Mater Today 13:24–30
Guillaume B (2016) Dental implants: a review. Morphologie 100:189–198
Chappard D, Terranova L, Mallet R, Mercier P (2015) 3D porous architecture of stacks of beta-TCP granules compared with that of trabecular bone: a microCT, vector analysis, and compression study. Front Endocrinol 6:161
Ndiaye M, Terranova L, Mallet R, Mabilleau G, Chappard D (2015) Three-dimensional arrangement of beta-tricalcium phosphate granules evaluated by microcomputed tomography and fractal analysis. Acta Biomater 11:404–411
Chappard D, Guillaume B, Mallet R, Pascaretti-Grizon F, Baslé MF, Libouban H (2010) Sinus lift augmentation and beta-TCP: a microCT and histologic analysis on human bone biopsies. Micron 41:321–326
Nyangoga H, Aguado E, Goyenvalle E, Baslé MF, Chappard D (2010) A non-steroidal anti-inflammatory drug (ketoprofen) does not delay beta-TCP bone graft healing. Acta Biomater 6:3310–3317
Lu J, Descamps M, Dejou J, Koubi G, Hardouin P, Lemaitre J, Proust JP (2002) The biodegradation mechanism of calcium phosphate biomaterials in bone. J Biomed Mater Res 63:408–412
Chazono M, Tanaka T, Kitasato S, Kikuchi T, Marumo K (2008) Electron microscopic study on bone formation and bioresorption after implantation of beta-tricalcium phosphate in rabbit models. J Orthop Sci 13:550–555
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7:292–304
Palmer JA, Abberton KM, Mitchell GM, Morrison WA (2014) Macrophage phenotype in response to implanted synthetic scaffolds: an immunohistochemical study in the rat. Cells Tissues Organs 199:169–183
Kajahn J, Franz S, Rueckert E, Forstreuter I, Hintze V, Moeller S, Simon JC (2012) Artificial extracellular matrices composed of collagen i and high sulfated hyaluronan modulate monocyte to macrophage differentiation under conditions of sterile inflammation. Biomatter 2:226–273
Londono R, Badylak SF (2015) Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng 43:577–592
Chen Z, Wu C, Gu W, Klein T, Crawford R, Xiao Y (2014) Osteogenic differentiation of bone marrow mscs by β-tricalcium phosphate stimulating macrophages via bmp2 signalling pathway. Biomaterials 35:1507–1518
Beuvelot J, Pascaretti-Grizon F, Filmon R, Moreau MF, Baslé MF, Chappard D (2011) In vitro assessment of osteoblast and macrophage mobility in presence of β-TCP particles by videomicroscopy. J Biomed Mater Res Part A 96:108–115
Li SP, Dai HL, Yan YH, Cao XY, Zheng QX (2005) Effect of macrophage on degradation of β-TCP ceramics. Key engineering materials. Trans Tech Publications, Zurich, pp 549–552
Xia Z, Zhu T, Du J, Zheng Q, Wang L, Li S, Chang C, Fang S (1994) Macrophages in degradation of collagen/hydroxylapatite (CHA), beta-tricalcium phosphate ceramics (TCP) artificial bone graft. An in vivo study. Chin Med J (Engl) 107:845–849
Detsch R, Schaefer S, Deisinger U, Ziegler G, Seitz H, Leukers B (2010) In vitro-osteoclastic activity studies on surfaces of 3D printed calcium phosphate scaffolds. J Biomater Appl 26:359–380
Choy J, Albers CE, Siebenrock KA, Dolder S, Hofstetter W, Klenke FM (2014) Incorporation of rankl promotes osteoclast formation and osteoclast activity on beta-TCP ceramics. Bone 69:80–88
Kucera T, Sponer P, Urban K, Kohout A (2014) Histological assessment of tissue from large human bone defects repaired with beta-tricalcium phosphate. Eur J Orthop Surg Traumatol 24:1357–1365
Schwartzwalder K, Somers H, Somers AV (1963) Method of making porous ceramics. In: 3090094 A, US Patent
Filmon R, Retailleau-Gaborit N, Brossard G, Grizon-Pascaretti F, Baslé MF, Chappard D (2009) Preparation of β-TCP granular material by polyurethane foam technology. Image Anal Stereol 28:1–10
Beebe K (2000) Alcohol/xylene: the unlikely fixative/dehydrant/clearant. J Histotechnol 23:45–50
Paschalis EP, Fratzl P, Gamsjaeger S, Hassler N, Brozek W, Eriksen EF, Rauch F, Glorieux FH, Shane E, Dempster D, Cohen A, Recker R, Klaushofer K (2015) Aging versus postmenopausal osteoporosis: bone composition and maturation kinetics at actively-forming trabecular surfaces of female subjects aged 1–84 years. J Bone Miner Res 31:347–357
Chappard D (2014) Technical aspects: How do we best prepare bone samples for proper histological analysis? In: Heymann D (ed) Bone cancer: progression and therapeutic approaches. Academic Press, Elsevier, London, pp 111–120
Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB (2003) Aging of microstructural compartments in human compact bone. J Bone Miner Res 18:1012–1019
Kazanci M, Fratzl P, Klaushofer K, Paschalis EP (2006) Complementary information on in vitro conversion of amorphous (precursor) calcium phosphate to hydroxyapatite from Raman microspectroscopy and wide-angle X-ray scattering. Calcif Tissue Int 79:354–359
Robey PG, Boskey AL (2009) The composition of bone. In: ASBMR (ed) Primer on the bone metabolic diseases and disorders of mineral metabolism, 7th edn. Wiley, Hoboken, pp 32–38
Pascaretti-Grizon F, Libouban H, Camprasse G, Camprasse S, Mallet R, Chappard D (2014) The interface between nacre and bone after implantation in the sheep: a nanotomographic and raman study. J Raman Spectrosc 45:558–564
Chiappetta N, Gruber B (2006) The role of mast cells in osteoporosis. Semin Arthritis Rheum 36:32–36
Saffar JL, Klapisz-Wolikow M (1990) Changes in mast cell number during the activation phase of an induced synchronized remodeling sequence in the rat. Bone 11:369–372
Baslé MF, Chappard D, Grizon F, Filmon R, Delecrin J, Daculsi G, Rebel A (1993) Osteoclastic resorption of ca-p biomaterials implanted in rabbit bone. Calcif Tissue Int 53:348–356
Zerbo IR, Zijderveld SA, de Boer A, Bronckers AL, de Lange G, ten Bruggenkate CM, Burger EH (2004) Histomorphometry of human sinus floor augmentation using a porous beta-tricalcium phosphate: a prospective study. Clin Oral Implant Res 15:724–732
Monchau F, Lefevre A, Descamps M, Belquin-Myrdycz A, Laffargue P, Hildebrand H (2002) In vitro studies of human and rat osteoclast activity on hydroxyapatite, β-tricalcium phosphate, calcium carbonate. Biomol Engin 19:143–152
Kotani S, Fujita Y, Kitsugi T, Nakamura T, Yamamuro T, Ohtsuki C, Kokubo T (1991) Bone bonding mechanism of beta-tricalcium phosphate. J Biomed Mater Res 25:1303–1315
Chiba S, Anada T, Suzuki K, Saito K, Shiwaku Y, Miyatake N, Baba K, Imaizumi H, Hosaka M, Itoi E (2016) Effect of resorption rate and osteoconductivity of biodegradable calcium phosphate materials on the acquisition of natural bone strength in the repaired bone. J Biomed Mater Res Part A 104:2833–2842
Gaasbeek RD, Toonen HG, van Heerwaarden RJ, Buma P (2005) Mechanism of bone incorporation of β-TCP bone substitute in open wedge tibial osteotomy in patients. Biomaterials 26:6713–6719
Terranova L, Libouban H, Mallet R, Chappard D (2015) Analysis of beta-tricalcium phosphate granules prepared with different formulations by nano-computed tomography and scanning electron microscopy. J Artif Organs 18:338–345
Aaron JE, Gallagher JC, Nordin BE (1974) Seasonal variation of histological osteomalacia in femoral-neck fractures. Lancet 2:84–85
Meunier PJ (1980) Bone biospy in diagnosis of metabolic bone disease. In: Cohn DV, Talmage RV, Matthews JL (eds) Hormonal control of bone metabolism. Proceedings of the 7th international conference calcium regulating hormones. Excerpta Medica, pp. 109-107
Aguado E, Pascaretti-Grizon F, Gaudin-Audrain C, Goyenvalle E, Chappard D (2014) Beta-TCP granules mixed with reticulated hyaluronic acid induce an increase in bone apposition. Biomed Mater 9:015001
Kuszyk BS, Heath DG, Bliss DF, Fishman EK (1996) Skeletal 3-d ct: advantages of volume rendering over surface rendering. Skeletal Radiol 25:207–214
Drebin RA, Carpenter L, Hanrahan P (1988) Volume rendering. ACM Siggraph Computer Graphics. ACM, New York, pp 65–74
Baslé MF, Bertrand G, Guyetant S, Chappard D, Lesourd M (1996) Migration of metal and polyethylene particles from articular prostheses may generate lymphadenopathy with histiocytosis. J Biomed Mater Res 30:157–163
Riihonen R, Supuran CT, Parkkila S, Pastorekova S, Väänänen HK, Laitala-Leinonen T (2007) Membrane-bound carbonic anhydrases in osteoclasts. Bone 40:1021–1031
Schaefer S, Detsch R, Uhl F, Deisinger U, Ziegler G (2011) How degradation of calcium phosphate bone substitute materials is influenced by phase composition and porosity. Adv Eng Mater 13:342–350
Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J (2005) Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175:342–349
Aparicio S, Doty S, Camacho N, Paschalis E, Spevak L, Mendelsohn R, Boskey A (2002) Optimal methods for processing mineralized tissues for Fourier transform infrared microspectroscopy. Calcif Tissue Int 70:422–429
Carson FL, Hladik C (2009) Fixation. In: Carson FL, Hladik C (eds) Histotechnology: A Self-Instructional Text. American Society for Clinical Pathology Press, Chicago, IL, pp 9–13
Strawn S, White J, Marshall G, Gee L, Goodis H, Marshall S (1996) Spectroscopic changes in human dentine exposed to various storage solutions—short term. J Dent 24:417–423
Mason JT, O’leary TJ (1991) Effects of formaldehyde fixation on protein secondary structure: a calorimetric and infrared spectroscopic investigation. J Histochem Cytochem 39:225–229
Pleshko NL, Boskey AL, Mendelsohn R (1992) An infrared study of the interaction of polymethyl methacrylate with the protein and mineral components of bone. J Histochem Cytochem 40:1413–1417
Pleshko NL, Boskey AL, Mendelsohn R (1992) An FT-IR microscopic investigation of the effects of tissue preservation on bone. Calcif Tissue Int 51:72–77
De Aza P, Santos C, Pazo A, De Aza S, Cusco R, Artus L (1997) Vibrational properties of calcium phosphate compounds. 1. Raman spectrum of β-tricalcium phosphate. Chem Mater 9:912–915
Kumar PN, Mishra SK, Kannan S (2015) Probing the limit of magnesium uptake by β-tricalcium phosphate in biphasic mixtures formed from calcium deficient apatites. J Solid State Chem 231:13–19
Quillard S, Paris M, Deniard P, Gildenhaar R, Berger G, Obadia L, Bouler J-M (2011) Structural and spectroscopic characterization of a series of potassium-and/or sodium-substituted β-tricalcium phosphate. Acta Biomater 7:1844–1852
Willis H, Zichy V, Hendra P (1969) The laser-raman and infra-red spectra of poly (methyl methacrylate). Polymer 10:737–746
Nabiev IR, Savchenko VA, Efremov ES (1983) Surface-enhanced raman spectra of aromatic amino acids and proteins adsorbed by silver hydrosols. J Raman Spectrosc 14:375–379
Frushour BG, Koenig JL (1975) Raman scattering of collagen, gelatin, and elastin. Biopolymers 14:379–391
Movasaghi Z, Rehman S, Rehman IU (2007) Raman spectroscopy of biological tissues. Appl Spectrosc Rev 42:493–541
Boer H, van Eek WH (1979) The penetration of the embedding medium methyl methacrylate in undecalcified bone. Microsc Acta 81:181–188
Mandair GS, Morris MD (2015) Contributions of Raman spectroscopy to the understanding of bone strength. Bonekey Rep 4:620
Gamsjaeger S, Masic A, Roschger P, Kazanci M, Dunlop JW, Klaushofer K, Paschalis EP, Fratzl P (2010) Cortical bone composition and orientation as a function of animal and tissue age in mice by Raman spectroscopy. Bone 47:392–399
Gullekson C, Lucas L, Hewitt K, Kreplak L (2011) Surface-sensitive Raman spectroscopy of collagen I fibrils. Biophys J 100:1837–1845
Acknowledgements
The authors thank Mrs Laurence Lechat for secretarial assistance, Mrs N. Retailleau and S. Lemière for microCT and histotechnology. E. Paschalis and G. Mabilleau are thanked for helpful discussions. This work was made possible by grants from ANR, program LabCom “NextBone”. The authors thank Kasios, 18 Chemin de la Violette, 31240 L’Union—France—for providing the β-TCP granules.
Author information
Authors and Affiliations
Contributions
The study was conceived by DC and BG. Surgery and patient handling were done by BG. Experimental analyses were designed by DC and performed by the acknowledged technicians. Histopathological analysis was done by DC. Raman analysis was done by FPG, SEM by BA, and nanoCT by LT and HL. The paper was written by DC and FPG, and the final manuscript was approved by all of the authors.
Corresponding author
Ethics declarations
Conflict of interest
Florence Pascaretti-Grizon, Bernard Guillaume, Lisa Terranova, Baptiste Arbez, Hélène Libouban, and Daniel Chappard declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
An informed consent was obtained from each subject. This experimental protocol was approved by the ethical committee of the French Collège d’Implantologie and was done in accordance with the institutional guidelines of the French Ethical Committee and with the 1964 Helsinki declaration and its later amendments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
223_2017_280_MOESM1_ESM.mp4
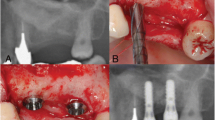
Supplementary material 1 Video movie of the block of the bone biopsy presented on Fig. 2A-B focusing on the interface between β-TCP granules (light grey) and bone trabeculae directly anchored on the granules (darker grey). In the marrow spaces, note the presence of numerous macrophages (in light grey) responsible of a “milky way” appearance. These phagocytic cells are revealed due to the presence of numerous β-TCP grains in their cytoplasm. (MP4 8028 kb)
Rights and permissions
About this article
Cite this article
Pascaretti-Grizon, F., Guillaume, B., Terranova, L. et al. Maxillary Sinus Lift with Beta-Tricalcium Phosphate (β-TCP) in Edentulous Patients: A Nanotomographic and Raman Study. Calcif Tissue Int 101, 280–290 (2017). https://doi.org/10.1007/s00223-017-0280-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0280-5