Abstract
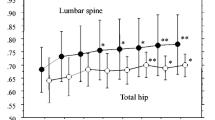
The aim of this study was to assess the long-term efficacy and safety of i.v. neridronate in the treatment of osteogenesis imperfecta (OI). One hundred and fourteen patients affected by OI were included in the study. Neridronate was administered by i.v. infusion at the dosage of 2 mg/kg, up to a maximum of 100 mg at three-month intervals for 3 years. Dual X-ray absorptiometry of the lumbar spine, hip, and ultradistal and proximal radius were evaluated every 6 months. Blood calcium, phosphate, albumin, fasting urinary calcium/creatinine ratio, total serum alkaline phosphatase, and bone alkaline phosphatase were obtained at baseline and every 3 months. The mean lumbar spine and total hip BMD significantly increased from baseline to any time point (p < 0.001). The mean ultradistal radius BMD significantly increased from baseline only at month 18 (p = 0.026), 30 (p = 0.046), and 36 (p = 0.013), respectively. The mean proximal radius BMD did not change during the whole observation. The levels of bone turnover markers significantly decreased from baseline to any post-baseline observation time. The study was not able to find any statistically significant effect on fracture risk (p = 0.185). The percentage of patients with fractures was unaltered during treatment as compared to the 3-year period before treatment. The most common AEs were fragility fractures, back pain, arthralgia, fever, and joint sprain. An acute phase reaction was reported in 26 (22.8%) patients. None of the reported SAEs were considered as treatment-related. Long-term treatment with i.v. neridronate has positive effects on BMD and bone turnover markers with a good safety profile, although no significant effect on the risk of fracture was observed.


Similar content being viewed by others
References
Forlino A, Marini JC (2016) Osteogenesis imperfecta. Lancet Lond Engl 387:1657–1671. doi:10.1016/S0140-6736(15)00728-X
Forlino A, Cabral WA, Barnes AM, Marini JC (2011) New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 7:540–557. doi:10.1038/nrendo.2011.81
Hoyer-Kuhn H, Netzer C, Semler O (2015) Osteogenesis imperfecta: pathophysiology and treatment. Wien Med Wochenschr 1946 165:278–284. doi:10.1007/s10354-015-0361-x
Baron R, Gertner JM, Lang R, Vignery A (1983) Increased bone turnover with decreased bone formation by osteoblasts in children with osteogenesis imperfecta tarda. Pediatr Res 17:204–207. doi:10.1203/00006450-198303000-00007
Braga V, Gatti D, Rossini M et al (2004) Bone turnover markers in patients with osteogenesis imperfecta. Bone 34:1013–1016. doi:10.1016/j.bone.2004.02.023
Cepollaro C, Gonnelli S, Pondrelli C et al (1999) Osteogenesis imperfecta: bone turnover, bone density, and ultrasound parameters. Calcif Tissue Int 65:129–132
Minisola S, Rosso R, Romagnoli E (1995) Bone resorption assessed by immunoassay of urinary cross-linked collagen peptides in patients with osteogenesis imperfecta. J Bone Miner Res 10:335–339. doi:10.1002/jbmr.5650100222
Brenner RE, Vetter U, Bollen AM et al (1994) Bone resorption assessed by immunoassay of urinary cross-linked collagen peptides in patients with osteogenesis imperfecta. J Bone Miner Res 9:993–997. doi:10.1002/jbmr.5650090706
Iwamoto J, Takeda T, Ichimura S (2002) Increased bone resorption with decreased activity and increased recruitment of osteoblasts in osteogenesis imperfecta type I. J Bone Miner Metab 20:174–179. doi:10.1007/s007740200025
Castells S (1973) New approaches to treatment of osteogenesis imperfecta. Clin Orthop 93:239–249
Marini JC, Bordenick S, Heavner G et al (1993) The growth hormone and somatomedin axis in short children with osteogenesis imperfecta. J Clin Endocrinol Metab 76:251–256. doi:10.1210/jcem.76.1.8421094
Antoniazzi F, Monti E, Venturi G, et al (2010) GH in combination with bisphosphonate treatment in osteogenesis imperfecta. Eur J Endocrinol 163:479–487. doi:10.1530/EJE-10-0208
Gatti D, Rossini M, Viapiana O et al (2013) Clinical development of neridronate: potential for new applications. Ther Clin Risk Manag 9:139–147. doi:10.2147/TCRM.S35788
Orwoll ES, Shapiro J, Veith S et al (2014) Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J Clin Invest 124:491–498. doi:10.1172/JCI71101
Horwitz EM, Prockop DJ, Gordon PL et al (2001) Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 97:1227–1231
Hoyer-Kuhn H, Semler O, Schoenau E (2014) Effect of denosumab on the growing skeleton in osteogenesis imperfecta. J Clin Endocrinol Metab 99:3954–3955. doi:10.1210/jc.2014-3072
Dwan K, Phillipi CA, Steiner RD, Basel D (2014) Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev 7:CD005088. doi:10.1002/14651858.CD005088.pub3
Adami S, Gatti D, Colapietro F et al (2003) Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res 18:126–130. doi:10.1359/jbmr.2003.18.1.126
Gatti D, Antoniazzi F, Prizzi R et al (2005) Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res 20:758–763. doi:10.1359/JBMR.041232
Hald JD, Evangelou E, Langdahl BL, Ralston SH (2015) Bisphosphonates for the prevention of fractures in osteogenesis imperfecta: meta-analysis of placebo-controlled trials. J Bone Miner Res 30:929–933. doi:10.1002/jbmr.2410
Adami S, Kanis JA (1995) Assessment of involutional bone loss: methodological and conceptual problems. J Bone Miner Res 10:511–517. doi:10.1002/jbmr.5650100402
Chevrel G, Schott A-M, Fontanges E et al (2006) Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3-year randomized placebo-controlled trial. J Bone Miner Res 21:300–306. doi:10.1359/JBMR.051015
Bradbury LA, Barlow S, Geoghegan F, et al (2012) Risedronate in adults with osteogenesis imperfecta type I: increased bone mineral density and decreased bone turnover, but high fracture rate persists. Osteoporos Int 23:285–294. doi:10.1007/s00198-011-1658-2
Folkestad L, Hald JD, Ersbøll AK et al (2016) Fracture rates and fracture sites in patients with osteogenesis imperfecta: A Nationwide Register-Based Cohort Study. J Bone Miner Res. doi:10.1002/jbmr.2920
Hennedige AA, Jayasinghe J, Khajeh J, Macfarlane TV (2013) Systematic review on the incidence of bisphosphonate related osteonecrosis of the jaw in children diagnosed with osteogenesis imperfecta. J Oral Maxillofac Res 4:e1. doi:10.5037/jomr.2013.4401
Maines E, Monti E, Doro F et al (2012) Children and adolescents treated with neridronate for osteogenesis imperfecta show no evidence of any osteonecrosis of the jaw. J Bone Miner Metab 30:434–438. doi:10.1007/s00774-011-0331-3
Nicolaou N, Agrawal Y, Padman M et al (2012) Changing pattern of femoral fractures in osteogenesis imperfecta with prolonged use of bisphosphonates. J Child Orthop 6:21–27. doi:10.1007/s11832-011-0380-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ombretta Viapiana has received speaking fees from Abiogen, Amgen, Merck Sharp & Dohme Corp. Luca Idolazzi has received speaking fees from Eli Lilly, UCB, Abbvie, Novartis. Angelo Fassio, Giovanni Orsolini, and Giovanni Adami declare they have no conflict of interest. Maurizio Rossini has received speaking and consulting fees from Abiogen, Amgen, Eli-Lilly, and Merck Sharp & Dohme Corp. Davide Gatti has received speaking fees from Abiogen, Amgen, Eli-Lilly, and Neopharmed-Gentili.
Human and Animal Rights and Informed Consent
The clinical study was conducted in accordance with the ethics principles of the Declaration of Helsinki and was approved by the local ethics committee. Each enrolled patient signed the informed consent to participate in the study.
Rights and permissions
About this article
Cite this article
Viapiana, O., Idolazzi, L., Fassio, A. et al. Long-term Effects of Neridronate in Adults with Osteogenesis Imperfecta: An Observational Three-Year Italian Study. Calcif Tissue Int 100, 341–347 (2017). https://doi.org/10.1007/s00223-017-0236-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0236-9