Abstract
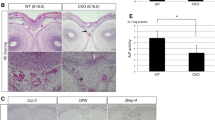
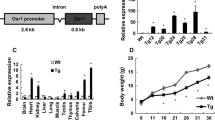
The Cre/loxP system has been widely used to generate tissue-specific gene knockout mice. Inducible (Tet-off) Osx-GFP::Cre (Osx-Cre) mouse line that targets osteoblasts is widely used in the bone research field. In this study, we investigated the effect of Osx-Cre on craniofacial bone development. We found that newborn Osx-Cre mice showed severe hypomineralization in parietal, frontal, and nasal bones as well as the coronal sutural area when compared to control mice. As the mice matured, the intramembranous bone hypomineralization phenotype became less severe. The major hypomineralization defect in parietal, frontal, and nasal bones had mostly disappeared by postnatal day 21, but the defect in sutural areas persisted. Importantly, Doxycycline treatment eliminated cranial bone defects at birth which indicates that Cre expression may be responsible for the phenotype. In addition, we showed that the primary calvarial osteoblasts isolated from neonatal Osx-Cre mice had comparable differentiation ability compared to their littermate controls. This study reinforces the idea that Cre-positive litter mates are indispensable controls in studies using conditional gene deletion.






Similar content being viewed by others
References
Liu F, Woitge HW, Braut A, Kronenberg MS, Lichtler AC, Mina M, Kream BE (2004) Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol 48:645–653
Rodda SJ, McMahon AP (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133:3231–3244
Dacquin R, Starbuck M, Schinke T, Karsenty G (2002) Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn 224:245–251
Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL (2002) Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem 277:44005–44012
Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schutz G, Tuckermann JP (2010) Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 11:517–531
Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J (2012) Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res 27:2344–2358
Razidlo DF, Whitney TJ, Casper ME, McGee-Lawrence ME, Stensgard BA, Li X, Secreto FJ, Knutson SK, Hiebert SW, Westendorf JJ (2010) Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS ONE 5:e11492
Wu JY, Aarnisalo P, Bastepe M, Sinha P, Fulzele K, Selig MK, Chen M, Poulton IJ, Purton LE, Sims NA, Weinstein LS, Kronenberg HM (2011) Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest 121:3492–3504
Zhu W, Liang G, Huang Z, Doty SB, Boskey AL (2011) Conditional inactivation of the CXCR4 receptor in osteoprecursors reduces postnatal bone formation due to impaired osteoblast development. J Biol Chem 286:26794–26805
Davey RA, Clarke MV, Sastra S, Skinner JP, Chiang C, Anderson PH, Zajac JD (2012) Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic Res 21:885–893
Schmidt-Supprian M, Rajewsky K (2007) Vagaries of conditional gene targeting. Nat Immunol 8:665–668
Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L, Goldstein SA, Krebsbach PH, Guan JL (2013) Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res 28:2414–2430
Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71
Liu F, Lee JY, Wei H, Tanabe O, Engel JD, Morrison SJ, Guan JL (2010) FIP200 is required for the cell-autonomous maintenance of fetal hematopoietic stem cells. Blood 116:4806–4814
McLeod MJ (1980) Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22:299–301
Forni PE, Scuoppo C, Imayoshi I, Taulli R, Dastru W, Sala V, Betz UA, Muzzi P, Martinuzzi D, Vercelli AE, Kageyama R, Ponzetto C (2006) High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly. J Neurosci 26:9593–9602
Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, Pu WT, Izumo S, Jay PY (2006) Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J Card Fail 12:392–398
Nishijima H, Yasunari T, Nakayama T, Adachi N, Shibahara K (2009) Improved applications of the tetracycline-regulated gene depletion system. Biosci Trends 3:161–167
Kuhnel F, Fritsch C, Krause S, Mundt B, Wirth T, Paul Y, Malek NP, Zender L, Manns MP, Kubicka S (2004) Doxycycline regulation in a single retroviral vector by an autoregulatory loop facilitates controlled gene expression in liver cells. Nucleic Acids Res 32:e30
Park JS, Baek WY, Kim YH, Kim JE (2011) In vivo expression of Osterix in mature granule cells of adult mouse olfactory bulb. Biochem Biophys Res Commun 407:842–847
Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, Maye P (2013) Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS ONE 8:e71318
Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F (2014) Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS ONE 9:e85161
Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ (1999) Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun 260:712–717
Huang WY, Aramburu J, Douglas PS, Izumo S (2000) Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat Med 6:482–483
Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR (2012) Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest 122:3101–3113
Acknowledgments
We thank Dr. Andrew McMahon for providing the Osx-Cre mice; Dr. Taylor Snider for critically reading and editing the manuscript. This study was funded by the National Institute Of Arthritis And Musculoskeletal And Skin Diseases of the National Institutes of Health under Award Number R01AR062030 (to F.L.) and R01DE020843 (to Y.M.).
Conflict of Interest
Dr. Fei Liu reports Grant from NIH during the conduct of the study. Dr. Yuji Mishina reports grants from NIH/NIDCR, personal fees from NIH/NICHD, others from NIH/NIEHS, personal fees from Shriners Hospital, grants from Department of Defense during the conduct of the study. Dr. Li Wang has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human subjects performed by any of the authors. All mice procedures used in this study were approved by the Institutional Animal Care and Use Committee at the University of Michigan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Mishina, Y. & Liu, F. Osterix-Cre Transgene Causes Craniofacial Bone Development Defect. Calcif Tissue Int 96, 129–137 (2015). https://doi.org/10.1007/s00223-014-9945-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9945-5