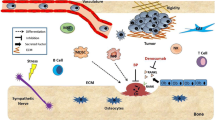
Abstract
Tumor cells with high skeletal homing affinity express numerous cell surface receptors that bind ligands produced in bone. Upon arrival, these cells survive in the host environment, encompassed in close proximity to bone marrow cells. Interactions between tumor cells and cells of the host microenvironment are essential to not only tumor cell survival but also their activation and proliferation into environment-modifying tumors. Through the production of RANKL, PTHrP, cytokines, and integrins, activated tumor cells stimulate osteoclastogenesis, enhance bone resorption, and subsequently release matrix-bound proteins that further promote tumor growth and bone resorption. In addition, alterations in the TGF-β/BMP and Wnt signaling pathways via tumor cell growth can either stimulate or suppress osteoblastic bone formation and function, leading to sclerotic or lytic bone disease, respectively. Hence, the presence of tumor cells in bone dysregulates bone remodeling, dramatically impairing skeletal integrity. Furthermore, through complex mechanisms, cells of the immune system interact with tumor cells to further impact bone remodeling. Lastly, with alterations in bone cell activity, the environment is permissive to promoting tumor growth further, suggesting an interdependence between tumor cells and bone cells in metastatic bone disease and multiple myeloma.


Similar content being viewed by others
References
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12:6243s–6249s
Townson JL, Chambers AF (2006) Dormancy of solitary metastatic cells. Cell Cycle 5:1744–1750
Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS (2003) Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem 89:462–473
Arya M, Patel HR, McGurk C, Tatoud R, Klocker H, Masters J, Williamson M (2004) The importance of the CXCL12-CXCR4 chemokine ligand–receptor interaction in prostate cancer metastasis. J Exp Ther Oncol 4:291–303
Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, Pienta KJ, Keller ET, McCauley LK, Taichman RS (2005) Skeletal localization and neutralization of the SDF-1 (CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res 20:318–329
Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS (2008) The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem 283:4283–4294
Kang H, Watkins G, Parr C, Douglas-Jones A, Mansel RE, Jiang WG (2005) Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res 7:R402–R410
Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3:537–549
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56
Vande Broek I, Leleu X, Schots R, Facon T, Vanderkerken K, Van Camp B, Van Riet I (2006) Clinical significance of chemokine receptor (CCR1, CCR2 and CXCR4) expression in human myeloma cells: the association with disease activity and survival. Haematologica 91:200–206
Zannettino AC, Farrugia AN, Kortesidis A, Manavis J, To LB, Martin SK, Diamond P, Tamamura H, Lapidot T, Fujii N, Gronthos S (2005) Elevated serum levels of stromal-derived factor-1alpha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res 65:1700–1709
Menu E, De Leenheer E, De Raeve H, Coulton L, Imanishi T, Miyashita K, Van Valckenborgh E, Van Riet I, Van Camp B, Horuk R, Croucher P, Vanderkerken K (2006) Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clin Exp Metastasis 23:291–300
Jung Y, Wang J, Song J, Shiozawa Y, Wang J, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS (2007) Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood 110:82–90
Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS (2008) Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem 105:370–380
Tamura D, Hiraga T, Myoui A, Yoshikawa H, Yoneda T (2008) Cadherin-11-mediated interactions with bone marrow stromal/osteoblastic cells support selective colonization of breast cancer cells in bone. Int J Oncol 33:17–24
Lee YC, Bilen MA, Yu G, Lin SC, Huang CF, Ortiz A, Cho H, Song JH, Satcher RL, Kuang J, Gallick GE, Yu-Lee LY, Huang W, Lin SH (2013) Inhibition of cell adhesion by an anti-cadherin 11 antibody prevents bone metastasis. Mol Cancer Res 29(8):430–435
Rudland PS, Platt-Higgins A, El-Tanani M, De Silva Rudland S, Barraclough R, Winstanley JH, Howitt R, West CR (2002) Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res 62:3417–3427
Thalmann GN, Sikes RA, Devoll RE, Kiefer JA, Markwalder R, Klima I, Farach-Carson CM, Studer UE, Chung LW (1999) Osteopontin: possible role in prostate cancer progression. Clin Cancer Res 5:2271–2277
Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN (2005) Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 106:1232–1239
Joseph J, Shiozawa Y, Jung Y, Kim JK, Pedersen E, Mishra A, Zalucha JL, Wang J, Keller ET, Pienta KJ, Taichman RS (2012) Disseminated prostate cancer cells can instruct hematopoietic stem and progenitor cells to regulate bone phenotype. Mol Cancer Res 10:282–292
Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121:1298–1312
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ (2000) RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA 97:1566–1571
Ehrlich LA, Roodman GD (2005) The role of immune cells and inflammatory cytokines in Paget’s disease and multiple myeloma. Immunol Rev 208:252–266
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillespie MT (1999) Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 140:4451–4458
Chen G, Sircar K, Aprikian A, Potti A, Goltzman D, Rabbani SA (2006) Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer 107:289–298
Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET (2001) Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest 107:1235–1244
Morrissey C, Kostenuik PL, Brown LG, Vessella RL, Corey E (2007) Host-derived RANKL is responsible for osteolysis in a C4-2 human prostate cancer xenograft model of experimental bone metastases. BMC Cancer 7:148
Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A, Brabbs AC, van Beek EJ, Holen I, Skerry TM, Dunstan CR, Russell GR, Van Camp B, Vanderkerken K (2001) Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood 98:3534–3540
Farrugia AN, Atkins GJ, To LB, Pan B, Horvath N, Kostakis P, Findlay DM, Bardy P, Zannettino AC (2003) Receptor activator of nuclear factor-kappaB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res 63:5438–5445
Sezer O, Heider U, Jakob C, Eucker J, Possinger K (2002) Human bone marrow myeloma cells express RANKL. J Clin Oncol 20:353–354
Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y (2001) Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA 98:11581–11586
Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S (2001) Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood 98:3527–3533
Shipman CM, Croucher PI (2003) Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res 63:912–916
Heath DJ, Vanderkerken K, Cheng X, Gallagher O, Prideaux M, Murali R, Croucher PI (2007) An osteoprotegerin-like peptidomimetic inhibits osteoclastic bone resorption and osteolytic bone disease in myeloma. Cancer Res 67:202–208
Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, Epstein J (2004) Cancer and the microenvironment: myeloma–osteoclast interactions as a model. Cancer Res 64:2016–2023
Canon JR, Roudier M, Bryant R, Morony S, Stolina M, Kostenuik PJ, Dougall WC (2008) Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis 25:119–129
Holland PM, Miller R, Jones J, Douangpanya H, Piasecki J, Roudier M, Dougall WC (2010) Combined therapy with the RANKL inhibitor RANK-Fc and rhApo2L/TRAIL/dulanermin reduces bone lesions and skeletal tumor burden in a model of breast cancer skeletal metastasis. Cancer Biol Ther 9:539–550
Miller RE, Roudier M, Jones J, Armstrong A, Canon J, Dougall WC (2008) RANK ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasis. Mol Cancer Ther 7:2160–2169
Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS, Coleman R, Paterson AH, Peterson MC, Fan M, Kinsey A, Jun S (2007) Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer–related bone metastases. J Clin Oncol 25:4431–4437
Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27:1564–1571
Liao J, McCauley LK (2006) Skeletal metastasis: established and emerging roles of parathyroid hormone related protein (PTHrP). Cancer Metastasis Rev 25:559–571
Guise TA (1997) Parathyroid hormone–related protein and bone metastases. Cancer 80:1572–1580
Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, Bennett RC, Martin TJ (1991) Localization of parathyroid hormone–related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res 51:3059–3061
Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR (1996) Evidence for a causal role of parathyroid hormone–related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 98:1544–1549
Johnson RW, Nguyen MP, Padalecki SS, Grubbs BG, Merkel AR, Oyajobi BO, Matrisian LM, Mundy GR, Sterling JA (2011) TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res 71:822–831
Lee SK, Lorenzo JA (1999) Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 140:3552–3561
Bryden AA, Hoyland JA, Freemont AJ, Clarke NW, George NJ (2002) Parathyroid hormone related peptide and receptor expression in paired primary prostate cancer and bone metastases. Br J Cancer 86:322–325
Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, Lentzsch S, Frank RC, Zweegman S, Wijermans PW, Orlowski RZ, Kranenburg B, Hall B, Casneuf T, Qin X, van de Velde H, Xie H, Thomas SK (2013) A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. British J Haematol 161:357–366
Adebanjo OA, Moonga BS, Yamate T, Sun L, Minkin C, Abe E, Zaidi M (1998) Mode of action of interleukin-6 on mature osteoclasts. Novel interactions with extracellular Ca2+ sensing in the regulation of osteoclastic bone resorption. J Cell Biol 142:1347–1356
Palmqvist P, Persson E, Conaway HH, Lerner UH (2002) IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappaB in mouse calvariae. J Immunol 169:3353–3362
Hobisch A, Rogatsch H, Hittmair A, Fuchs D, Bartsch G Jr, Klocker H, Bartsch G, Culig Z (2000) Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J Pathol 191:239–244
Siegall CB, Schwab G, Nordan RP, FitzGerald DJ, Pastan I (1990) Expression of the interleukin 6 receptor and interleukin 6 in prostate carcinoma cells. Cancer Res 50:7786–7788
Knupfer H, Schmidt R, Stanitz D, Brauckhoff M, Schonfelder M, Preiss R (2004) CYP2C and IL-6 expression in breast cancer. Breast 13:28–34
Kinder M, Chislock E, Bussard KM, Shuman L, Mastro AM (2008) Metastatic breast cancer induces an osteoblast inflammatory response. Exp Cell Res 314:173–183
Sethi N, Dai X, Winter CG, Kang Y (2011) Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19:192–205
Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S, Bonomini S, Hojden M, Sammarelli G, Barille S, Bataille R, Rizzoli V (2002) Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood 100:4615–4621
Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, Grano M, Colucci S, Svaldi M, Rizzoli V (2005) Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood 106:2472–2483
Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ (2003) Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 33:28–37
Markovina S, Callander NS, O’Connor SL, Xu G, Shi Y, Leith CP, Kim K, Trivedi P, Kim J, Hematti P, Miyamoto S (2010) Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-kappaB activity in myeloma cells. Mol Cancer 9:176
Wright LM, Maloney W, Yu X, Kindle L, Collin-Osdoby P, Osdoby P (2005) Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone 36:840–853
Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD (2001) Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood 97:3349–3353
Abe M, Hiura K, Wilde J, Moriyama K, Hashimoto T, Ozaki S, Wakatsuki S, Kosaka M, Kido S, Inoue D, Matsumoto T (2002) Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood 100:2195–2202
Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S, Grubbs B, Zhao M, Chen D, Sherry B, Mundy GR (2003) Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood 102:311–319
DuPre SA, Redelman D, Hunter KW Jr (2007) The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumors and metastatic tumor foci. Int J Exp Pathol 88:351–360
Duong LT, Lakkakorpi P, Nakamura I, Rodan GA (2000) Integrins and signaling in osteoclast function. Matrix Biol 19:97–105
Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Lu X, Haffty BG, Pantel K, Massague J, Kang Y (2011) VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20:701–714
Nannuru KC, Futakuchi M, Varney ML, Vincent TM, Marcusson EG, Singh RK (2010) Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor–bone interface. Cancer Res 70:3494–3504
Bonfil RD, Dong Z, Trindade Filho JC, Sabbota A, Osenkowski P, Nabha S, Yamamoto H, Chinni SR, Zhao H, Mobashery S, Vessella RL, Fridman R, Cher ML (2007) Prostate cancer–associated membrane type 1-matrix metalloproteinase: a pivotal role in bone response and intraosseous tumor growth. Am J Pathol 170:2100–2111
Lynch CC (2011) Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone 48:44–53
Thiolloy S, Halpern J, Holt GE, Schwartz HS, Mundy GR, Matrisian LM, Lynch CC (2009) Osteoclast-derived matrix metalloproteinase-7, but not matrix metalloproteinase-9, contributes to tumor-induced osteolysis. Cancer Res 69:6747–6755
Divella R, Daniele A, Savino E, Palma F, Bellizzi A, Giotta F, Simone G, Lioce M, Quaranta M, Paradiso A, Mazzocca A (2013) Circulating levels of transforming growth factor-beta (TGF-beta) and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of distant seeding of circulating tumor cells in patients with metastatic breast cancer. Anticancer Res 33:1491–1497
McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM (2006) Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem 281:24171–24181
Zhang Z, Hu Z, Gupta J, Krimmel JD, Gerseny HM, Berg AF, Robbins JS, Du H, Prabhakar B, Seth P (2012) Intravenous administration of adenoviruses targeting transforming growth factor beta signaling inhibits established bone metastases in 4T1 mouse mammary tumor model in an immunocompetent syngeneic host. Cancer Gene Ther 19:630–636
Feeley BT, Gamradt SC, Hsu WK, Liu N, Krenek L, Robbins P, Huard J, Lieberman JR (2005) Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. J Bone Miner Res 20:2189–2199
Barnes J, Anthony CT, Wall N, Steiner MS (1995) Bone morphogenetic protein-6 expression in normal and malignant prostate. World J Urol 13:337–343
Lee YC, Cheng CJ, Bilen MA, Lu JF, Satcher RL, Yu-Lee LY, Gallick GE, Maity SN, Lin SH (2011) BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res 71:5194–5203
Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET (2005) Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res 65:8274–8285
Seckinger A, Meissner T, Moreaux J, Goldschmidt H, Fuhler GM, Benner A, Hundemer M, Reme T, Shaughnessy JD Jr, Barlogie B, Bertsch U, Hillengass J, Ho AD, Pantesco V, Jauch A, De Vos J, Rossi JF, Mohler T, Klein B, Hose D (2009) Bone morphogenic protein 6: a member of a novel class of prognostic factors expressed by normal and malignant plasma cells inhibiting proliferation and angiogenesis. Oncogene 28:3866–3879
Schwaninger R, Rentsch CA, Wetterwald A, van der Horst G, van Bezooijen RL, van der Pluijm G, Lowik CW, Ackermann K, Pyerin W, Hamdy FC, Thalmann GN, Cecchini MG (2007) Lack of noggin expression by cancer cells is a determinant of the osteoblast response in bone metastases. Am J Pathol 170:160–175
Rentsch CA, Cecchini MG, Thalmann GN (2009) Loss of inhibition over master pathways of bone mass regulation results in osteosclerotic bone metastases in prostate cancer. Swiss Med Weekly 139:220–225
Secondini C, Wetterwald A, Schwaninger R, Thalmann GN, Cecchini MG (2011) The role of the BMP signaling antagonist noggin in the development of prostate cancer osteolytic bone metastasis. PloS One 6:e16078
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131:324–336
Leto G, Incorvaia L, Badalamenti G, Tumminello FM, Gebbia N, Flandina C, Crescimanno M, Rini G (2006) Activin A circulating levels in patients with bone metastasis from breast or prostate cancer. Clin Exp Metastasis 23:117–122
Terpos E, Kastritis E, Christoulas D, Gkotzamanidou M, Eleutherakis-Papaiakovou E, Kanellias N, Papatheodorou A, Dimopoulos MA (2012) Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann Oncol 23:2681–2686
Vallet S, Mukherjee S, Vaghela N, Hideshima T, Fulciniti M, Pozzi S, Santo L, Cirstea D, Patel K, Sohani AR, Guimaraes A, Xie W, Chauhan D, Schoonmaker JA, Attar E, Churchill M, Weller E, Munshi N, Seehra JS, Weissleder R, Anderson KC, Scadden DT, Raje N (2010) Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci USA 107:5124–5129
Nicks KM, Perrien DS, Akel NS, Suva LJ, Gaddy D (2009) Regulation of osteoblastogenesis and osteoclastogenesis by the other reproductive hormones, activin and inhibin. Mol Cell Endocrinol 310:11–20
Chantry AD, Heath D, Mulivor AW, Pearsall S, Baud’huin M, Coulton L, Evans H, Abdul N, Werner ED, Bouxsein ML, Key ML, Seehra J, Arnett TR, Vanderkerken K, Croucher P (2010) Inhibiting activin-A signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. J Bone Miner Res 25:2633–2646
Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr (2003) The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349:2483–2494
Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, Rabbani SA (2004) Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer 101:1345–1356
Hall CL, Kang S, MacDougald OA, Keller ET (2006) Role of Wnts in prostate cancer bone metastases. J Cell Biochem 97:661–672
Li ZG, Yang J, Vazquez ES, Rose D, Vakar-Lopez F, Mathew P, Lopez A, Logothetis CJ, Lin SH, Navone NM (2008) Low-density lipoprotein receptor-related protein 5 (LRP5) mediates the prostate cancer-induced formation of new bone. Oncogene 27:596–603
Thudi NK, Martin CK, Murahari S, Shu ST, Lanigan LG, Werbeck JL, Keller ET, McCauley LK, Pinzone JJ, Rosol TJ (2011) Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate 71:615–625
Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET (2005) Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res 65:7554–7560
Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, Hayward SW, Cunha GR, Marker PC (2005) Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res 65:10423–10430
Roato I, D’Amelio P, Gorassini E, Grimaldi A, Bonello L, Fiori C, Delsedime L, Tizzani A, De Libero A, Isaia G, Ferracini R (2008) Osteoclasts are active in bone forming metastases of prostate cancer patients. PloS One 3:e3627
Voorzanger-Rousselot N, Goehrig D, Journe F, Doriath V, Body JJ, Clezardin P, Garnero P (2007) Increased Dickkopf-1 expression in breast cancer bone metastases. Br J Cancer 97:964–970
Bu G, Lu W, Liu CC, Selander K, Yoneda T, Hall C, Keller ET, Li Y (2008) Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer 123:1034–1042
Dai J, Hall CL, Escara-Wilke J, Mizokami A, Keller JM, Keller ET (2008) Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res 68:5785–5794
Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, Croucher PI, Terpos E (2006) Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer 19:1728–1731
Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Donofrio G, Bonomini S, Sala R, Mangoni M, Rizzoli V (2007) Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res 67:7665–7674
Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC (2009) Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114:371–379
Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T (2005) Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood 106:3160–3165
Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD Jr (2008) Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone 42:669–680
Fowler JA, Mundy GR, Lwin ST, Edwards CM (2012) Bone marrow stromal cells create a permissive microenvironment for myeloma development: a new stromal role for Wnt inhibitor Dkk1. Cancer Res 72:2183–2189
Kim Y, Reifenberger G, Lu D, Endo T, Carson DA, Gast SM, Meschenmoser K, Nowak M, Schmidt-Wolf IG (2011) Influencing the Wnt signaling pathway in multiple myeloma. Anticancer Res 31:725–730
Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr (2007) Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood 109:2106–2111
Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD Jr, Evans HR, Snowden JA, Stover DR, Vanderkerken K, Croucher PI (2009) Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res 24:425–436
Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B, Mundy GR (2008) Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood 111:2833–2842
Colucci S, Brunetti G, Oranger A, Mori G, Sardone F, Specchia G, Rinaldi E, Curci P, Liso V, Passeri G, Zallone A, Rizzi R, Grano M (2011) Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J 1:e27
Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, Delimpasi S, Pouli A, Meletis J, Kastritis E, Zervas K, Dimopoulos MA (2012) Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer 131:1466–1471
Gkotzamanidou M, Dimopoulos MA, Kastritis E, Christoulas D, Moulopoulos LA, Terpos E (2012) Sclerostin: a possible target for the management of cancer-induced bone disease. Expert Opin Ther Targets 16:761–769
Mendoza-Villanueva D, Zeef L, Shore P (2011) Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFbeta-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res 13:R106
Meyers KE, Sethna C (2013) Endothelin antagonists in hypertension and kidney disease. Pediatr Nephrol 28:711–720
Clines GA, Mohammad KS, Grunda JM, Clines KL, Niewolna M, McKenna CR, McKibbin CR, Yanagisawa M, Suva LJ, Chirgwin JM, Guise TA (2011) Regulation of postnatal trabecular bone formation by the osteoblast endothelin A receptor. J Bone Miner Res 26:2523–2536
Guise TA, Mohammad KS (2004) Endothelins in bone cancer metastases. Cancer Treat Res 118:197–212
Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, Simons JW (1995) Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med 1:944–949
Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA (2003) A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci USA 100:10954–10959
Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, Sleep DJ, Isaacson JD, Nelson JB, Atrasentan Phase III, Institutions Study Group (2007) A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 110:1959–1966
James ND, Caty A, Borre M, Zonnenberg BA, Beuzeboc P, Morris T, Phung D, Dawson NA (2009) Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebo-controlled, randomised, phase 2 trial. Eur Urol 55:1112–1123
Tokuda H, Hanai Y, Matsushima-Nishiwaki R, Yamauchi J, Doi T, Harada A, Takai S, Kozawa O (2007) Rho-kinase regulates endothelin-1-stimulated IL-6 synthesis via p38 MAP kinase in osteoblasts. Biochem Biophys Res Commun 362:799–804
Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB (2000) The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene 19:6043–6052
Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS (2001) EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res 61:2301–2306
Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, Iwakura Y, Suda T, Matsuo K (2009) Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem 284:14637–14644
Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD Jr, Barlogie B, Yaccoby S (2009) The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood 114:1803–1812
Coltrera MD, Wang J, Porter PL, Gown AM (1995) Expression of platelet-derived growth factor B-chain and the platelet-derived growth factor receptor beta subunit in human breast tissue and breast carcinoma. Cancer Res 55:2703–2708
Seymour L, Dajee D, Bezwoda WR (1993) Tissue platelet-derived growth factor (PDGF) predicts for shortened survival and treatment failure in advanced breast cancer. Breast Cancer Res Treat 26:247–252
Canalis E, Rydziel S (1996) Platelet-derived growth factor and the skeleton. In: Bilezikian JB, Raisz LG, Rodan A (eds) Principles of bone biology. Academic Press, San Diego, CA, pp 619–626
Vordemvenne T, Paletta JR, Hartensuer R, Pap T, Raschke MJ, Ochman S (2011) Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC Musculoskelet Disord 12:263
Franchimont N, Canalis E (1995) Platelet-derived growth factor stimulates the synthesis of interleukin-6 in cells of the osteoblast lineage. Endocrinology 136:5469–5475
Hamdan R, Zhou Z, Kleinerman ES (2011) SDF-1alpha induces PDGF-B expression and the differentiation of bone marrow cells into pericytes. Mol Cancer Res 9:1462–1470
Xue Y, Lim S, Yang Y, Wang Z, Jensen LD, Hedlund EM, Andersson P, Sasahara M, Larsson O, Galter D, Cao R, Hosaka K, Cao Y (2012) PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med 18:100–110
Valta MP, Hentunen T, Qu Q, Valve EM, Harjula A, Seppanen JA, Vaananen HK, Harkonen PL (2006) Regulation of osteoblast differentiation: a novel function for fibroblast growth factor 8. Endocrinology 147:2171–2182
Kitagawa Y, Dai J, Zhang J, Keller JM, Nor J, Yao Z, Keller ET (2005) Vascular endothelial growth factor contributes to prostate cancer–mediated osteoblastic activity. Cancer Res 65:10921–10929
DeNardo DG, Andreu P, Coussens LM (2010) Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev 29:309–316
Noonan K, Marchionni L, Anderson J, Pardoll D, Roodman GD, Borrello I (2010) A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood 116:3554–3563
Zhao E, Wang L, Dai J, Kryczek I, Wei S, Vatan L, Altuwaijri S, Sparwasser T, Wang G, Keller ET, Zou W (2012) Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology 1:152–161
Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, Ponnazhagan S (2012) Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol 189:4258–4265
Zirakzadeh AA, Marits P, Sherif A, Winqvist O (2013) Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J Immunol 190:5847–5855
Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH, Rosenblatt JD (2005) Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer 117:574–586
Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, Schwartz SM, Pascual V, Hood LE, Clark EA (1998) OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol 161:6113–6121
Youn JI, Gabrilovich DI (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40:2969–2975
Danilin S, Merkel AR, Johnson JR, Johnson RW, Edwards JR, Sterling JA (2012) Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology 1:1484–1494
Zhuang J, Zhang J, Lwin ST, Edwards JR, Edwards CM, Mundy GR, Yang X (2012) Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells. PloS One 7:e48871
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kovacic, N., Croucher, P.I. & McDonald, M.M. Signaling Between Tumor Cells and the Host Bone Marrow Microenvironment. Calcif Tissue Int 94, 125–139 (2014). https://doi.org/10.1007/s00223-013-9794-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-013-9794-7