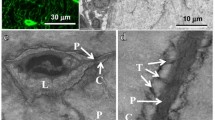
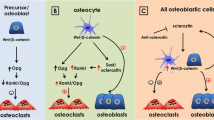
Abstract
Osteocytes, the most abundant cells in bone, have been long postulated to detect and respond to mechanical and hormonal stimuli and to coordinate the function of osteoblasts and osteoclasts. The discovery that the inhibitor of bone formation sclerostin is primarily expressed in osteocytes in bone and downregulated by anabolic stimuli provided a mechanism by which osteocytes influence the activity of osteoblasts. Advances of the last few years provided experimental evidence demonstrating that osteocytes also participate in the recruitment of osteoclasts and the initiation of bone remodeling. Apoptotic osteocytes trigger yet-to-be-identified signals that attract osteoclast precursors to specific areas of bone, which in turn differentiate to mature, bone-resorbing osteoclasts. Osteocytes are also the source of molecules that regulate the generation and activity of osteoclasts, such as OPG and RANKL; and genetic manipulations of the mouse genome leading to loss or gain of function or to altered expression of either molecule in osteocytes markedly affect bone resorption. This review highlights these investigations and discusses how the novel concept of osteocyte-driven bone resorption and formation impacts our understanding of the mechanisms by which current therapies control bone remodeling.




Similar content being viewed by others
References
Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S et al (2006) E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol 26:4539–4552
Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M et al (1999) MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99:81–92
Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, Chrysovergis K et al (2005) The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci 118:147–156
Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC (1999) Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 104:439–446
Boyce BF, Xing L, Jilka RL, Bellido T, Weinstein RS, Parfitt AM, Manolagas SC (2002) Apoptosis in bone cells. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology. Academic Press, San Diego, pp 151–168
Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L et al (2009) Identification of differentially expressed genes between osteoblasts and osteocytes. Bone 45:682–692
Igwe JC, Gao Q, Kizivat T, Kao WW, Kalajzic I (2011) Keratocan is expressed by osteoblasts and can modulate osteogenic differentiation. Connect Tissue Res 52:401–407
Benson MD, Aubin JE, Xiao G, Thomas PE, Franceschi RT (1999) Cloning of a 2.5 kb murine bone sialoprotein promoter fragment and functional analysis of putative Osf2 binding sites. J Bone Miner Res 14:396–405
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ et al (2010) Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 30:3071–3085
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238
Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC et al (2005) Chronic elevation of PTH in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583
Wang L, Ciani C, Doty SB, Fritton SP (2004) Delineating bone’s interstitial fluid pathway in vivo. Bone 34:499–509
Poole KE, Van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Van Bezooijen RL, Roelen BA, Visser A, Wee-Pals L, de Wilt E, Karperien M, Hamersma H et al (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D et al (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C et al (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543
Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE et al (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68:577–589
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C et al (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y et al (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 24:1651–1661
Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D et al (2005) Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 15:928–935
Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, Olivos N et al (2011) PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling. J Bone Miner Res 26:1035–1046
Warmington K, Morony S, Sarosi I, Gong G, Stepphens P, Winkler DG, Sutherland MK et al (2004) Sclerostin antagonism in adult rodents, via monoclonal antibody mediated blockade, increases bone mineral density and implicates sclerostin as a key regulator of bone mass during adulthood. J Bone Miner Res 19:S56
Warmington K, Ominsky M, Bolon B, Cattley R, Stephens P, Lawson A, Lightwood D et al (2005) Sclerostin monoclonal antibody treatment of osteoporotic rats completely reverses one year of ovariectomy-induced systemic bone loss. J Bone Miner Res 20:S22
Paszty C, Turner CH, Robinson MK (2010) Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res 25:1897–1904
Jilka RL (2009) Inhibiting the inhibitor: a new route to bone anabolism. J Bone Miner Res 24:575–577
Keller H, Kneissel M (2005) SOST is a target gene for PTH in bone. Bone 37:148–158
van Lierop AH, Witteveen J, Hamdy N, Papapoulos S (2010) Patients with primary hyperparathyroidism have lower circulating sclerostin levels than euparathyroid controls. Eur J Endocrinol 163:833–837
Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D et al (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062
Mirza FS, Padhi ID, Raisz LG, Lorenzo JA (2010) Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab 95:1991–1997
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MJ, Alam I, Mantila SM et al (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M et al (2012) Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50:209–217
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, Bonewald LF et al (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241
Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T (2006) Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res 21:605–615
Honma M, Ikebuchi Y, Kariya Y, Hayashi M, Hayashi N, Aoki S, Suzuki H (2013) RANKL subcellular trafficking and regulatory mechanisms in osteocytes. J Bone Miner Res doi. doi:10.1002/jbmr.1941
Bellido T, Saini V, Pajevic PD (2013) Effects of PTH on osteocyte function. Bone 54:250–257
Rhee Y, Allen MR, Condon K, Plotkin LI, Lezcano V, Vyas K, O’Brien CA et al (2009) PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling: divergent role of Sost and the Wnt pathway. J Bone Miner Res 24:S78
O’Brien CA, Plotkin LI, Galli C, Goellner J, Gortazar AR, Allen MR, Robling AG et al (2008) Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One 3:e2942
Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J et al (2012) Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res 27:1018–1029
Harris SE, MacDougall M, Horn D, Woodruff K, Zimmer SN, Rebel VI, Fajardo R et al (2012) Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone 50:42–53
Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J et al (2003) Mechanical loading: biphasic osteocyte survival and the targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol 284:C934–C943
Bellido T, Plotkin LI (2011) Novel actions of bisphosphonates in bone: preservation of osteoblast and osteocyte viability. Bone 49:50–55
Jilka RL, Bellido T, Almeida M, Plotkin LI, O’Brien CA, Weinstein RS, Manolagas SC (2008) Apoptosis in bone cells. In: Bilezikian JP, Raisz LG, Martin TJ (eds) Principles of bone biology. Academic Press, San Diego, pp 237–261
Tomkinson A, Reeve J, Shaw RW, Noble BS (1997) The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab 82:3128–3135
Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS (1998) The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res 13:1243–1250
Huber C, Collishaw S, Mosley JR, Reeve J, Noble BS (2007) Selective estrogen receptor modulator inhibits osteocyte apoptosis during abrupt estrogen withdrawal: implications for bone quality maintenance. Calcif Tissue Int 81:139–144
Mann V, Huber C, Kogianni G, Collins F, Noble B (2007) The antioxidant effect of estrogen and selective estrogen receptor modulators in the inhibition of osteocyte apoptosis in vitro. Bone 40:674–684
Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K et al (2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730
Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O’Brien CA, Plotkin LI et al (2002) Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298:843–846
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest 102:274–282
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S et al (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297
Bellido T (2010) Antagonistic interplay between mechanical forces and glucocorticoids in bone: a tale of kinases. J Cell Biochem 111:1–6
Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T (1999) Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 104:1363–1374
Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T (2005) Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases and ERKs. Am J Physiol Cell Physiol 289:C633–C643
Bakker A, Klein-Nulend J, Burger E (2004) Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem Biophys Res Commun 320:1163–1168
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615
Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE (2007) Wnt/β-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor α. J Biol Chem 282:20715–20727
Sunters A, Armstrong VJ, Zaman G, Kypta RM, Kawano Y, Lanyon LE, Price JS (2010) Mechano-transduction in osteoblastic cells involves strain-regulated, estrogen receptor α-mediated, control of IGF-IR sensitivity to ambient IGF, leading to PI3-K/ AKT dependent, Wnt/LRP5 receptor-independent activation of β-catenin signaling. J Biol Chem 285:8743–8758
Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S (2005) Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem 280:41342–41351
Gortazar AR, Martin-Millan M, Bravo B, Plotkin LI, Bellido T (2013) Crosstalk between caveolin-1/extracellular signal–regulated kinase (ERK) and ß-catenin survival pathways in osteocyte mechanotransduction. J Biol Chem 288:8168–8175
Verborgt O, Gibson G, Schaffler MB (2000) Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 15:60–67
Verborgt O, Tatton NA, Majeska RJ, Schaffler MB (2002) Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res 17:907–914
Bellido T (2007) Osteocyte apoptosis induces bone resorption and impairs the skeletal response to weightlessness. Bonekey Osteovision 4:252–256
Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M et al (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 5:464–475
Yang J, Shah R, Robling AG, Templeton E, Yang H, Tracey KJ, Bidwell JP (2008) HMGB1 is a bone-active cytokine. J Cell Physiol 214:730–739
Jilka RL, Noble B, Weinstein RS (2013) Osteocyte apoptosis. Bone 54:264–271
Kogianni G, Mann V, Noble BS (2008) Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localised bone destruction. J Bone Miner Res 23:915–927
Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB (2012) Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 50:1115–1122
Marcus R (2002) Mechanisms of exercise effects on bone. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology. Academic Press, San Diego, pp 1477–1488
Bikle DD, Halloran BP, Morey-Holton E (1997) Spaceflight and the skeleton: lessons for the earthbound. Gravit Space Biol Bull 10:119–135
Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC (2003) Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest 111:1651–1664
Plotkin LI, Manolagas SC, Bellido T (2002) Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem 277:8648–8657
Plotkin LI, Bellido T (2001) Bisphosphonate-induced, hemichannel-mediated, anti-apoptosis through the Src/ERK pathway: a gap junction–independent action of connexin43. Cell Adhes Commun 8:377–382
Parfitt AM (2002) Life history of osteocytes: relationship to bone age, bone remodeling, and bone fragility. J Musculoskelet Neuronal Interact 2:499–500
Parfitt AM (2002) Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone 30:5–7
Manolagas SC, Parfitt AM (2010) What old means to bone. Trends Endocrinol Metab 21:369–374
Manolagas SC (2010) From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300
Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O’Brien CA, Thostenson J et al (2009) Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in 21-month-old mice. Aging Cell 9:147–161
Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun L, Rhee Y et al (2012) Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res 27:374–389
Plotkin LI, Bellido T (2013) Beyond gap junctions: connexin43 and bone cell signaling. Bone 52:157–166
Zhang Y, Paul EM, Sathyendra V, Davidson A, Bronson S, Srinivasan S, Gross TS et al (2011) Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One 6:e23516
Grimston SK, Brodt MD, Silva MJ, Civitelli R (2008) Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1). J Bone Miner Res 23:879–886
Qiu S, Rao DS, Palnitkar S, Parfitt AM (2002) Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone 31:313–318
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y et al (2006) WNT/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281:31720–31728
Acknowledgments
This research was supported by the National Institutes of Health (R01-AR053643, KO2-AR02127, R03 TW006919, R01-DK076007, and P01-AG13918).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
The author has stated that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Bellido, T. Osteocyte-Driven Bone Remodeling. Calcif Tissue Int 94, 25–34 (2014). https://doi.org/10.1007/s00223-013-9774-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-013-9774-y