Abstract
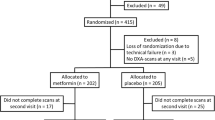
Factors that contribute to bone fragility in type 2 diabetes are not well understood. We assessed the effects of intensive glycemic control, thiazolidinediones (TZDs), and A1C levels on bone geometry and strength at the radius and tibia. In a substudy of the Action to Control Cardiovascular Risk in Diabetes trial, peripheral quantitative computed tomographic (pQCT) scans of the radius and tibia were obtained 2 years after randomization on 73 participants (intensive n = 35, standard n = 38). TZD use and A1C levels were measured every 4 months during the trial. Effects of intervention assignment, TZD use, and A1C on pQCT parameters were assessed in linear regression models. Intensive, compared with standard, glycemic control was associated with 1.3 % lower cortical volumetric BMD at the tibia in men (p = 0.02) but not with other pQCT parameters. In women, but not men, each additional year of TZD use was associated with an 11 % lower polar strength strain index (SSIp) at the radius (p = 0.04) and tibia (p = 0.002) in models adjusted for A1C levels. In women, each additional 1 % increase in A1C was associated with an 18 % lower SSIp at the ultradistal radius (p = 0.04) in models adjusted for TZD use. There was no consistent evidence of an effect of intensive, compared with standard, glycemic control on bone strength at the radius or tibia. In women, TZD use may reduce bone strength at these sites. Higher A1C may also be associated with lower bone strength at the radius, but not tibia, in women.
Similar content being viewed by others
References
Schwartz AV, Margolis KL, Sellmeyer DE, Vittinghoff E, Ambrosius WT, Bonds DE, Josse RG, Schnall AM, Simmons DL, Hue TF, Palermo L, Hamilton BP, Green JB, Atkinson HH, O’Connor PJ, Force RW, Bauer DC (2012) Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care 35:1525–1531
Loke YK, Singh S, Furberg CD (2009) Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 180:32–39
Douglas IJ, Evans SJ, Pocock S, Smeeth L (2009) The risk of fractures associated with thiazolidinediones: a self-controlled case-series study. PLoS Med 6:e1000154
Hsiao FY, Mullins CD (2010) The association between thiazolidinediones and hospitalisation for fracture in type 2 diabetic patients: a Taiwanese population-based nested case-control study. Diabetologia 53:489–496
Dormuth CR, Carney G, Carleton B, Bassett K, Wright JM (2009) Thiazolidinediones and fractures in men and women. Arch Intern Med 169:1395–1402
Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M (2010) Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab 12:716–721
Colhoun HM, Livingstone SJ, Looker HC, Morris AD, Wild SH, Lindsay RS, Reed C, Donnan PT, Guthrie B, Leese GP, McKnight J, Pearson DW, Pearson E, Petrie JR, Philip S, Sattar N, Sullivan FM, McKeigue P (2012) Hospitalised hip fracture risk with rosiglitazone and pioglitazone use compared with other glucose-lowering drugs. Diabetologia 55:2929–2937
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 18:427–444
Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, Yu Q, Zillikens MC, Gao X, Rivadeneira F (2012) Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol 27:319–332
Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC Jr, Grimm RH Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD (2007) Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 99:21i–33i
Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559
Ferretti JL, Capozza RF, Zanchetta JR (1996) Mechanical validation of a tomographic (pQCT) index for noninvasive estimation of rat femur bending strength. Bone 18:97–102
Liu D, Manske SL, Kontulainen SA, Tang C, Guy P, Oxland TR, McKay HA (2007) Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int 18:991–997
Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC Jr, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER, Action to Control Cardiovascular Risk in Diabetes I (2010) Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 33:983–990
Schwartz AV, Vittinghoff E, Margolis KL, Ambrosius WT, Bonds DE, Josse RG, Sellmeyer DE, Schnall AM, Simmons DL, Palermo L, Force RW, Green JB, O’Connor PJ, Bauer DC (2011) Intensive glycemic control and fracture risk. Diabetes 60:A372
Muller ME, Webber CE, Bouxsein ML (2003) Predicting the failure load of the distal radius. Osteoporos Int 14:345–352
Gorai I, Nonaka K, Kishimoto H, Sakata H, Fujii Y, Fujita T (2001) Cut-off values determined for vertebral fracture by peripheral quantitative computed tomography in Japanese women. Osteoporos Int 12:741–748
Sheu Y, Zmuda JM, Boudreau RM, Petit MA, Ensrud KE, Bauer DC, Gordon CL, Orwoll ES, Cauley JA (2011) Bone strength measured by peripheral quantitative computed tomography and the risk of nonvertebral fractures: the Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res 26:63–71
Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B (2004) Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145:401–406
Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, Strotmeyer ES, Resnick HE, Carbone L, Beamer BA, Park SW, Lane NE, Harris TB, Cummings SR (2006) Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab 91:3349–3354
Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR (2007) The peroxisome-proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 92:1305–1310
Glintborg D, Andersen M, Hagen C, Heickendorff L, Hermann AP (2008) Association of pioglitazone treatment with decreased bone mineral density in obese premenopausal patients with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab 93:1696–1701
Berberoglu Z, Yazici AC, Demirag NG (2010) Effects of rosiglitazone on bone mineral density and remodelling parameters in postmenopausal diabetic women: a 2-year follow-up study. Clin Endocrinol (Oxf) 73:305–312
Borges JL, Bilezikian JP, Jones-Leone AR, Acusta AP, Ambery PD, Nino AJ, Grosse M, Fitzpatrick LA, Cobitz AR (2011) A randomized, parallel group, double-blind, multicentre study comparing the efficacy and safety of Avandamet (rosiglitazone/metformin) and metformin on long-term glycaemic control and bone mineral density after 80 weeks of treatment in drug-naive type 2 diabetes mellitus patients. Diabetes Obes Metab 13:1036–1046
Bilezikian JP, Eastell R, Nino AJ, Northcutt AR, Kravitz B, Paul G, Cobitz AR, Josse RG, Fitzpatrick LA (2011) Rosiglitazone reduced BMD in women with type 2 diabetes and the effect was attenuated after switching to metformin. J Bone Miner Res 26:S68–S69
Syversen U, Stunes AK, Gustafsson BI, Obrant KJ, Nordsletten L, Berge R, Thommesen L, Reseland JE (2009) Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr Disord 9:10
Sardone LD, Renlund R, Willett TL, Fantus IG, Grynpas MD (2011) Effect of rosiglitazone on bone quality in a rat model of insulin resistance and osteoporosis. Diabetes 60:3271–3278
Stunes AK, Westbroek I, Gustafsson BI, Fossmark R, Waarsing JH, Eriksen EF, Petzold C, Reseland JE, Syversen U (2011) The peroxisome proliferator–activated receptor (PPAR) alpha agonist fenofibrate maintains bone mass, while the PPAR gamma agonist pioglitazone exaggerates bone loss, in ovariectomized rats. BMC Endocr Disord 11:11
Broulik PD, Sefc L, Haluzik M (2011) Effect of PPAR-gamma agonist rosiglitazone on bone mineral density and serum adipokines in C57BL/6 male mice. Folia Biol (Praha) 57:133–138
Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR (2008) Use of thiazolidinediones and fracture risk. Arch Intern Med 168:820–825
Kanazawa I, Yamaguchi T, Yamamoto M, Sugimoto T (2010) Relationship between treatments with insulin and oral hypoglycemic agents versus the presence of vertebral fractures in type 2 diabetes mellitus. J Bone Miner Metab 28:554–560
Yaturu S, Bryant B, Jain SK (2007) Thiazolidinediones treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care 30:1574–1576
Gregorio F, Cristallini S, Santeusanio F, Filipponi P, Fumelli P (1994) Osteopenia associated with non-insulin-dependent diabetes mellitus: what are the causes? Diabetes Res Clin Pract 23:43–54
McCabe LR (2007) Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem 102:1343–1357
Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, Masharani UB, Schwartz AV, Li X, Link TM (2012) Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging 35:117–124
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615
Garcia-Martin A, Rozas-Moreno P, Reyes-Garcia R, Morales-Santana S, Garcia-Fontana B, Garcia-Salcedo JA, Munoz-Torres M (2012) Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 97:234–241
Noble BS, Reeve J (2000) Osteocyte function, osteocyte death and bone fracture resistance. Mol Cell Endocrinol 159:7–13
Vogt MT, Cauley JA, Kuller LH, Nevitt MC (1997) Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res 12:283–289
Strotmeyer ES, Cauley JA, Schwartz AV, de Rekeneire N, Resnick HE, Zmuda JM, Shorr RI, Tylavsky FA, Vinik AI, Harris TB, Newman AB (2006) Reduced peripheral nerve function is related to lower hip BMD and calcaneal QUS in older white and black adults: the health, aging, and body composition study. J Bone Miner Res 21:1803–1810
Ishani A, Paudel M, Taylor BC, Barrett-Connor E, Jamal S, Canales M, Steffes M, Fink HA, Orwoll E, Cummings SR, Ensrud KE (2008) Renal function and rate of hip bone loss in older men: the Osteoporotic fractures in men study. Osteoporos Int 19:1549–1556
Acknowledgments
The ACCORD BONE ancillary study was funded by a grant (R01DK069514) from the National Institute of Diabetes and Digestive and Kidney Diseases. GlaxoSmithKline provided support for the pQCT substudy. The ACCORD study was supported by Grants (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010) from the National Heart, Lung, and Blood Institute; by other components of the National Institutes of Health, including the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Eye Institute; by the Centers for Disease Control and Prevention; and by General Clinical Research Centers. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, Omron Healthcare, Sanofi-Aventis, and Schering-Plough.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Ensrud has a consultant/advisory role for Merck Sharpe & Dohme. A. Schwartz has a consultant/advisory role for and has received funding from Glaxo SmithKline. L. Palermo has a consultant/advisory role for Nycomed. All other authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Schwartz, A.V., Vittinghoff, E., Margolis, K.L. et al. Intensive Glycemic Control and Thiazolidinedione Use: Effects on Cortical and Trabecular Bone at the Radius and Tibia. Calcif Tissue Int 92, 477–486 (2013). https://doi.org/10.1007/s00223-013-9703-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-013-9703-0