Abstract
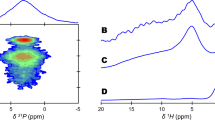
Water is well recognized as an important component in bone, typically regarded as a constituent of collagen, a pore-filling fluid in bone, and an adsorbed species on the surface of bone crystallites. The possible siting and role of water within the structure of the apatite crystallites have not been fully explored. In our experiments, carbonated hydroxyl- and fluorapatites were prepared in D2O and characterized by elemental analysis, thermal gravimetric analysis, powder X-ray diffraction, and infrared and Raman spectroscopy. Two hydroxylapatites and two fluorapatites, with widely different amounts of carbonate were analyzed by solid state 2H NMR spectroscopy using the quadrupole echo pulse sequence, and each spectrum showed one single line as well as a low-intensity powder pattern. The relaxation time of 7.1 ms for 5.9 wt% carbonated hydroxylapatite indicates that the single line is likely due to rapid, high-symmetry jumps in translationally rigid D2O molecules, indicative of structural incorporation within the lattice. Discrimination between structurally incorporated and adsorbed water is enhanced by the rapid exchange of surface D2O with atmospheric H2O. Moreover, a 2H resonance was observed for samples dried under a variety of conditions, including in vacuo heating to 150°C. In contrast, a sample heated to 500°C produced no deuterium resonance, indicating that structural water had been released by that temperature. We propose that water is located in the c-axis channels. Because structural water is observed even for apatites with very low carbonate content, some of the water molecules must lie between the monovalent ions.







Similar content being viewed by others
References
Roseberry HH, Hastings AB, Morse JK (1931) X-ray analysis of bone and teeth. J Biol Chem 90:395–407
Beevers CA, McIntyre DB (1946) The atomic structure of fluor-apatite and its relation to that of tooth and bone material. Miner Mag 27:254–257
McConnell D (1962) The crystal structure of bone. Clin Orthopaedics 23:253–268
Elliott JC (2002) Calcium phosphate biominerals. In: Kohn MJ, Rakovan J, Hughes JM (eds) Phosphates—geochemical, geobiological, and materials importance. Reviews in mineralogy and geochemistry 48. Mineralogical Society of America, Chantilly, pp 427–453
Pan Y, Fleet ME (2002) Compositions of the apatite-group minerals: substitution mechanisms and controlling factors. In: Kohn MJ, Rakovan J, Hughes JM (eds) Phosphates—geochemical, geobiological, and materials importance. Reviews in mineralogy and geochemistry 48. Mineralogical Society of America, Chantilly, pp 13–49
LeGeros RZ, Trautz OR, Klein E, LeGeros JP (1969) Two types of carbonate substitution in the apatite structure. Experientia 25:5–7
Elliott JC (1994) Structure and chemistry of the apatites and other calcium orthophosphates. Elsevier, New York
Fleet ME, Liu X (2007) Coupled substitution of type A and B carbonate in sodium-bearing apatite. Biomaterials 28:916–926
Cheng ZH, Yasukawa A, Kandori K, Ishikawa T (1998) FTIR study on incorporation of CO2 into calcium hydroxyapatite. J Chem Soc Faraday Trans 94:1501–1505
Ishikawa T, Termachi A, Hidekazu T, Yasukawa A, Kandori K (2000) Fourier transform infrared spectroscopy of deuteration of calcium hydroxyapatite particles. Langmuir 16:10221–10226
Joris SJ, Amberg CH (1971) The nature of the deficiency in nonstoichiometric hydroxyapatite. II. Spectroscopic studies of calcium and strontium hydroxyapatite. J Phys Chem 75:3172–3178
McConnell D (1970) Crystal chemistry of bone mineral: hydrated carbonate apatites. Am Miner 55:1659–1669
Simpson DR (1964) The nature of alkali carbonate apatites. Am Miner 49:363–376
Biltz RM, Pellegrino ED (1971) The hydroxyl content of calcified tissue mineral. Calcif Tissue Int 36:259–263
LeGeros RZ, Bonel G, Legros R (1978) Types of “H2O” in human enamel and in precipitated apatites. Calcif Tissue Int 26:111–118
Ivanova TI, Frank-Kamenetaskaya OV, Kol’tsov AB, Ugolkov VL (2001) Crystal structure of calcium-deficient carbonated hydroxyapatite. Thermal decomposition. J Solid State Chem 160:340–349
Wilson RM, Dowker SEP, Elliott JC (2006) Rietveld refinements and spectroscopic structural studies of a Na-free carbonate apatite made by hydrolysis of monetite. Biomaterials 27:4682–4692
Wilson RM, Elliott JC, Dowker SEP, Smith RI (2004) Rietveld structure refinement of precipitated carbonate apatite using neutron diffraction data. Biomaterials 25:2205–2213
Yesinowski J, Eckert H (1987) Hydrogen environments in calcium phosphates-1H MAS NMR at high spin speeds. J Am Chem Soc 109:6274–6282
Kaflak-Hachulska A, Samoson A, Kolodziejski W (2003) 1H MAS and 1H CP/MAS NMR study of human bone mineral. Calcif Tissue Int 73:476–486
Santos RA, Wind RA, Bronnimann CE (1994) 1H CRAMPS and 1H–31P Hetcor experiments on bone, bone mineral, and model calcium phosphate phases. J Magn Reson B 105:183–187
Kolmas J, Kolodziejski W (2007) Concentration of hyroxyl groups in dental apatites: a solid-state 1H MAS NMR study using inverse 31P → 1H cross-polarization. Chem Commun (Camb) 42:4390–4392
Sfihi H, Rey C (2002) 1-D and 2-D double heteronuclear magnetic resonance study of the local structure of type B carbonate fluoroapatite. In: Fraissard J, Lapina B (eds) Magnetic resonance in colloid and interface science. NATO ASI Series II, vol 76. Kluwer Academic, New York, pp 409–422
Jäger C, Welzel T, Meyer-Zalka W, Epple M (2006) A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn Reson Chem 44:573–580
Cho G, Wu Y, Ackerman JL (2003) Detection of hydroxyl ions in bone mineral by solid-state NMR spectroscopy. Science 300:1123–1127
Sandström DE, Jarlbring M, Antzutkin ON, Forsling W (2006) A spectroscopic study of calcium surface sites and adsorbed iron species at aqueous fluorapatite by means of 1H and 31P MAS NMR. Langmuir 22:11060–11064
Jarlbring M, Sandström DE, Antzutkin ON, Forsling W (2006) Characterization of active phosphorus surface sites at synthetic carbonate-free fluorapatite using single-pulse 1H, 31P, and 31P CP MAS NMR. Langmuir 22:4787–4792
Rey C, Combes C, Drouet C, Sfihi H, Barroug A (2007) Physico-chemical properties of nanocrystalline apatites: implications for biominerals and biomaterials. Mater Sci Eng 27:198–205
Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MMJ, Beck LW (2006) Three structural roles for water in bone observed by solid-state NMR. Biophys J 90:3722–3731
Mason HE, McCubbin FM, Smirnov A, Phillips BL (2009) Solid-state NMR and IR spectroscopic investigation of the role of structural water and F in carbonate-rich fluorapatite. Am Miner 94:507–516
Reyes-Casga J, Garcia-Garcia R, Arellano-Jimenez MJ, Sanchez-Pastenes E, Tiznado-Orozco GE, Gill-Chavarria IM, Gomez-Casga G (2008) Structural and thermal behaviour of human tooth and three synthetic hydroxyapaties from 20 to 600°C. J Phys D Appl Phys 41:225407–225418
Jonas K, Vassanyi I, Ungvari I (1980) The study of synthetic carbonate–hydroxyapatites and dental enamels by IR and derivatographic methods. Phys Chem Miner 6:55–60
Kaflak A, Kolodziejski W (2011) Complimentary information on water and hydroxyl groups in nanocrystalline carbonated hydroxyapatites from TGA NMR and IR measurements. J Mol Struct 990:263–270
Rey C, Combes C, Drouet C, Glimcher MJ (2009) Bone mineral: update on chemical composition and structure. Osteoporosis Int 20:1013–1021
Mitchell PCH, Parker SF, Simkiss K, Simmons J, Taylor MG (1996) Hydrated sites in biogenic amorphous calcium phosphates: an infrared, Raman, and inelastic neutron scattering study. J Inorg Biochem 62:83–187
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C Mater Biol Appl 25:131–143
Casciani FS (1971) Identification of hydrate water in enamel, dentine, cementum, and bone. In: Fearnhead RW, Stack MV (eds) Tooth Enamel II. John Wright & Sons, Bristol
Wittebort RJ, Usha MG, Ruben DJ, Wemmer DE, Pines A (1988) Observation of molecular reorientation in ice by proton and deuterium magnetic resonance. J Am Chem Soc 110:5668–5671
Benesi AJ, Gruktzeck MW, O’Hare B, Phair JW (2005) Room-temperature icelike water in kanemite detected by 2H NMR T1 relaxation. Langmuir 21:527–529
Weiss A, Weiden N (1980) Deuteron magnetic resonance in crystal hydrates. In: Smith JAS (ed) Advances in nuclear quadrupole resonance, vol 4. Heyden & Sons, London
Reeves LW (1969) The study of water in hydrate crystals by nuclear magnetic resonance. In: Emsley JW, Feeney J, Sutcliffe LH (eds) Progress in NMR spectroscopy, vol 4. Pergammon Press, Oxford
Benesi AJ, Grutzeck MW, O’Hare B, Phair JW (2004) Room temperature solid surface water with tetrahedral jumps of 2H nuclei detected in 2H2O hydrated porous silicates. J Phys Chem B 108:17783–17790
Wilson RM, Elliott JC, Dowker SEP (2003) Formate incorporation in the structure of Ca-deficient apatite: Rietveld structure refinement. J Solid State Chem 174:132–140
Greenfield DJ, Termine JD, Eanes ED (1974) A chemical study of apatites prepared by hydrolysis of amorphous calcium phosphates in carbonate-containing aqueous solutions. Calcif Tissue Int 14:131–138
Wu Y, Ackerman JL, Kim H-M, Rey C, Barroug A, Glimcher MJ (2002) Nuclear magnetic resonance spin-spin relaxation of the crystals of bone, dental enamel, and synthetic hydroxyapatites. J Bone Miner Res 17:472–480
De Maeyer EAP, Verbeeck RMH, Naessens DE (1993) Stoichiometry of Na+- and CO3 2−-containing apatites obtained by hydrolysis of monetite. Inorg Chem 32:5709–5714
Krajewski A, Mazzocchi M, Buldini PL, Ravaglioli A, Tinti A, Taddei P, Fagnano C (2005) Synthesis of carbonated hydroxyapatites: efficiency of the substitution and critical evaluation of analytical methods. J Mol Struct 744–747:221–228
Graziano G (2006) Water: cavity size distribution and hydrogen bonds. Chem Phys Lett 396:226–231
Acknowledgments
The authors are indebted to Dr. Alan Benesi of Pennsylvania State University for acquisition of NMR spectra and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yoder, C.H., Pasteris, J.D., Worcester, K.N. et al. Structural Water in Carbonated Hydroxylapatite and Fluorapatite: Confirmation by Solid State 2H NMR. Calcif Tissue Int 90, 60–67 (2012). https://doi.org/10.1007/s00223-011-9542-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-011-9542-9