Abstract
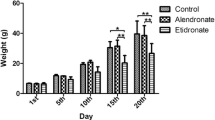
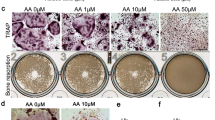
Osteoclast differentiation and functioning are strictly controlled by RANKL expressed on osteoblast membrane surfaces, but whether osteoclasts exert control over osteoblasts remains unclear. In the present study, we examined the effect of an osteoclast inhibitor, a bisphosphonate (BP), on the response of maxillary bone to mechanical stress in a high-turnover osteoporosis model (OPG−/− mice, a model of juvenile Paget disease). Mechanical stress was induced by use of orthodontic elastics to move the maxillary first molar. BP was administered once per day beginning 5 days before elastic insertion. Relative to wild type (WT), in the OPG−/− mice tooth movement distance was greater, resorption of the interradicular septum occurred to a greater extent, the osteoclast count was higher, and serum alkaline phosphatase (ALP) was higher. However, administration of BP to OPG−/− mice reduced tooth movement distance, increased bone volume at the interradicular septum, decreased the osteoclast count, and reduced serum ALP. BP administration also caused a temporal shift in peak Runx2 staining in OPG−/− mice, such that the overall staining time course was similar to that observed for WT mice. We conclude that BP administration not only inhibited osteoclast activity in OPG−/− mice but also systemically and locally inhibited osteoblast activity. It is possible that osteoclasts are able to exert some negative control over osteoblasts.






Similar content being viewed by others
References
Sato Y, Sakai H, Kobayashi Y, Shibasaki Y, Sasaki T (2000) Bisphosphonate administration alters subcellular localization of vacuolar-type H+-ATPase and cathepsin K in osteoclasts during experimental movement of rat molars. Anat Rec 260:72–80
Sato Y, Shibasaki Y, Sasaki T (2000) Effects of bisphosphonate administration on root and bone resorption during experimental movement of rat molars. In: Davidovitch Z, Mah J (eds) Biological mechanisms of tooth movement and craniofacial adaptation. EBSCO Media, Birmingham, pp 243–252
Chung HS, Sasaki T, Sato Y, Shibasaki Y (1999) H+-ATPase inhibitor, bafilomycin A1, reduces bone resorption during experimental movement of rat molars. Orthodont Waves 58:183–192
Yokoya K, Sasaki T, Shibasaki Y (1997) Distributional changes of osteoclasts and preosteoclastic cells in periodontal tissues during experimental tooth movement as revealed by quantitative immunohistochemistry of H+-ATPase. J Dent Res 76:580–587
Suda T, Takahasi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357
Kong YY, Yashida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424
Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ (2000) RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA 97:1566–1571
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chan MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS (1998) Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12:1260–1268
Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashino K, Ozawa H (1998) Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 247:610–615
Whyte MP, Obrecht SE, Finnegan PM, Jones JL, Podgornik MN, McAlister WH, Mumm S (2002) Osteoprotegerin deficiency and juvenile Paget disease. N Engl J Med 347:175–184
Cundy T, Hegde M, Naot D, Chong B, King A, Wallace R, Mulley J, Love DR, Seidel J, Fawkner M, Banovic T, Callon KE, Grey AB, Reid IR, Middleton-Hardie CA, Cornish J (2002) A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum Mol Genet 11:2119–2127
Cundy T, Wheadon L, King A (2004) Treatment of idiopathic hyperphosphatasia with intensive bisphosphonate therapy. J Bone Miner Res 19:703–711
Antoniades K, Karakasis D, Kapetanos G, Lasaridis N, Tzarou V (1993) Chronic idiopathic hyperphosphatasemia. Case report. Oral Surg Oral Med Oral Pathol 76:200–204
Golob DS, McAlister WH, Mills BG, Fedde KN, Reinus WR, Teitelbaum SL, Beeki S, Whyte MP (1996) Juvenile Paget disease: life-long features of a mildly affected young woman. J Bone Miner Res 11:132–142
Whyte MP, Singhellakis PN, Petersen MB, Davies M, Totty WG, Mumm S (2007) Juvenile Paget disease: the second reported, oldest patient is homozygous for the TNFRSF11B “Balkan” mutation (966_969delTGACinsCTT), which elevates circulating immunoreactive osteoprotegerin levels. J Bone Miner Res 22:938–946
Tau C, Mautalen C, Casco C, Alvarez V, Rubinstein M (2004) Chronic idiopathic hyperphosphatasia: normalization of bone turnover with cyclical intravenous pamidronate therapy. Bone 35:210–216
Mitsudo SM (1971) Chronic idiopathic hyperphosphatasia associated with pseudoxanthoma elasticum. J Bone Joint Surg Am 53:303–314
Fretzin DF (1975) Pseudoxanthoma elasticum in hyperphosphatasia. Arch Dermatol 111:271–272
Amizuka N, Shimomura J, Li M, Seki Y, Oda K, Henderson JE, Mizuno A, Ozawa H, Maeda T (2003) Defective bone remodelling in osteoprotegerin-deficient mice. J Electron Microsc 52:503–513
Nakamura M, Udagawa N, Matsuura S, Mogi M, Nakamura H, Horiuchi H, Saito N, Hiraoka BY, Kobayashi Y, Takaoka K, Ozawa H, Miyazawa H, Takahashi N (2003) Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology 144:5441–5446
Kanzaki S, Ito M, Takada Y, Ogawa K, Matsuo K (2006) Resorption of auditory ossicles and hearing loss in mice lacking osteoprotegerin. Bone 39:414–419
Zehnder AF, Kristiansen AG, Adams JC, Kujawa SG, Merchant SN, McKenna MJ (2006) Osteoprotegrin knockout mice demonstrate abnormal remodeling of the otic capsule and progressive hearing loss. Laryngoscope 116:201–206
Kimura M, Miyazawa K, Tabuchi M, Maeda H, Kameyama Y, Goto S (2008) Bisphosphonate treatment increases the size of the mandibular condyle and normalizes growth of the mandibular ramus in osteoprotegerin-deficient mice. Calcif Tissue Int 82:137–147
Komori T (2005) Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem 95:445–453
Watanabe T, Okafuji N, Nakano K, Shimizu T, Muraoka R, Kurihara S, Yamada K, Kawakami T (2007) Periodontal tissue reaction to mechanical stress in mice. J Hard Tissue Biol 16:71–74
Watanabe T, Nakano N, Muraoka R, Shimizu T, Okafuji N, Kurihara S, Yamada K, Kawakami T (2008) Role of Msx2 as a promoting factor for Runx2 at the periodontal tension sides elicited by mechanical stress. Eur J Med Res 13:425–431
Tabuchi M, Miyazawa K, Kimura M, Maeda H, Kawai T, Kameyama Y, Goto S (2005) Enhancement of crude morphogenetic protein-induced new bone formation and normalization of endochondral ossification by bisphosphonate treatment in osteoprotegerin-deficient mice. Calcif Tissue Int 77:239–249
Sprogar S, Vaupotic T, Cör A, Drevensek M, Drevensek G (2008) The endothelin system mediates bone modeling in the late stage of orthodontic tooth movement in rats. Bone 43:740–747
Wu LC, D’Amelio F, Fox RA, Polyakov I, Daunton NG (1997) Light microscopic image analysis system to quantify immunoreactive terminal area apposed to nerve cells. J Neurosci Methods 74:89–96
Yoshimatsu M, Uehara M, Yoshida N (2008) Expression of heat shock protein 47 in the periodontal ligament during orthodontic tooth movement. Arch Oral Biol 53:890–895
Kimmel DB, Jee WSS (1983) Measurements of area, perimeter, and distance: details of data collection in bone histomorphometry. In: Recker RR (ed) Bone histomorphometry. Techniques and interpretation. CRC Press, Boca Raton, pp 89–108
Yamashiro T, Takano-Yamamoto T (2001) Influences of ovariectomy on experimental tooth movement in the rat. J Dent Res 80:1858–1861
Verna C, Dalstra M, Melsen B (2000) The rate and the type of orthodontic tooth movement is influenced by bone turnover in a rat model. Eur J Orthod 22:343–352
Freisch H (1998) Bisphosphonates: mechanisms of action. Endocr Rev 19:80–100
Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS (2004) Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 25:4105–4115
Miyazono K, Hellman U, Wenstedt C, Helden C-H (1988) Latent high molecular weight complex of transforming growth factor β1. J Biol Chem 263:6407–6415
Hosoi T, Asuka T, Motto M, Tomita T, Shiraki M, Inoue S, Ouchi Y, Orimo H (1996) Immunolocalization of transforming growth factor-β in the bone tissue. Calcif Tissue Int 59:305–306
Matsunobu T, Torigoe K, Ishikawa M, de Vega S, Kulkarni AB, Iwamoto Y, Yamada Y (2009) Critical roles of the TGF-beta type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Dev Biol 332:325–338
Acknowledgements
We thank Dr. Takeyasu Maeda and Dr. Minqi Li of Niigata University for instructing us in the method of elastic insertion. This study was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (21592618).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shoji, S., Tabuchi, M., Miyazawa, K. et al. Bisphosphonate Inhibits Bone Turnover in OPG−/− Mice Via a Depressive Effect on Both Osteoclasts and Osteoblasts. Calcif Tissue Int 87, 181–192 (2010). https://doi.org/10.1007/s00223-010-9384-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9384-x