Abstract
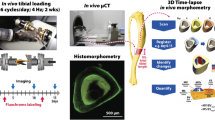
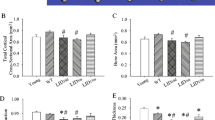
With aging, the skeleton may have diminished responsiveness to mechanical stimulation. The senescence-accelerated mouse SAMP6 has many features of senile osteoporosis and is thus a useful model to examine how the osteoporotic skeleton responds to mechanical loading. We performed in vivo tibial bending on 4-month-old SAMP6 (osteoporotic) and SAMR1 (control) mice. Loading was applied daily (60 cycles/day, 5 days/week) for 2 weeks at peak force levels that produced estimated endocortical strains of 1,000 and 2,000 με. In a separate group of mice, sham bending was applied. Comparisons were made between right (loaded) and left (nonloaded) tibiae. Tibial bone marrow cells were cultured under osteogenic conditions and stained for alkaline phosphatase (ALP) and alizarin red (ALIZ) at 14 and 28 days, respectively. Tibiae were then embedded in plastic and sectioned, and endocortical bone formation was assessed based on calcein labels. Tibial bending did not alter the osteogenic potential of the marrow as there were no significant differences in ALP or ALIZ staining between loaded and nonloaded bones. Tibial bending activated the formation of endocortical bone in both SAMP6 and SAMR1 mice, whereas sham bending did not elicit an endocortical response. Both groups of mice exhibited bending strain-dependent increases in bone formation rate. We found little evidence of diminished responsiveness to loading in the SAMP6 skeleton. In conclusion, the ability of the SAMP6 mouse to respond normally to an anabolic mechanical stimulus distinguishes it from chronologically aged animals. This finding highlights a limitation of the SAMP6 mouse as a model of senile osteoporosis.




Similar content being viewed by others
References
Riggs BL, Melton LJ (1983) Evidence for two distinct syndromes of involutional osteoporosis. Am J Med 75:899–901
Kassem M, Melton LJ, Riggs BL (1996) The type I/type II model for involutional osteoporosis. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis. Academic Press, San Diego, pp 691–702
Lips P, Courpron P, Meunier PJ (1978) Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res 26:13–17
Darby AJ, Meunier PJ (1981) Mean wall thickness and formation periods of trabecular bone packets in idiopathic osteoporosis. Calcif Tissue Int 33:199–204
Parfitt AM, Villanueva AR, Foldes J, Rao DS (1995) Relations between histologic indices of bone formation: implications for the pathogenesis of spinal osteoporosis. J Bone Miner Res 10:466–473
Recker RR, Kimmel DB, Parfitt AM, Davies KM, Keshawarz N, Hinders S (1988) Static and tetracycline-based bone histomorphometric data from 34 normal postmenopausal females. J Bone Miner Res 3:133–144
Clarke BL, Ebeling PR, Jones JD, Wahner HW, O’Fallon WM, Riggs BL, Fitzpatrick LA (1996) Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab 81:2264–2270
Friedenstein AJ (1976) Precursor cells of mechanocytes. Int Rev Cytol 47:327–359
Beresford JN (1989) Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Rel Res 240:270–280
Gimble JM, Robinson CE, Wu X, Kelly KA (1996) The function of adipocytes in the bone marrow stroma: an update. Bone 19:421–428
Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ (1996) Age-related changes in osteogenic stem cells in mice. J Bone Miner Res 11:568–577
Kahn A, Gibbons R, Perkins S, Gazit D (1995) Age-related bone loss: a hypothesis and initial assessment in mice. Clin Orthop Rel Res 313:69–75
D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA (1999) Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 14:1115–1122
Mueller SM, Glowacki J (2001) Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem 82:583–590
Stenderup K, Justesen J, Clausen C, Kassem M (2003) Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33:919–926
Sethe S, Scutt A, Stolzing A (2006) Aging of mesenchymal stem cells. Ageing Res Rev 5:91–116
Rubin CT, Bain SD, McCleod KJ (1992) Suppression of osteogenic response in the aging skeleton. Calcif Tissue Int 50:306–313
Turner CH, Takano Y, Owan I (1995) Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res 10:1544–1549
Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS (2003) Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone 33:946–955
Klein-Nulend J, Sterck JGH, Semeins CM, Lips P, Joldersma M, Baart JA, Burger EH (2002) Donor age and mechanosensitivity of human bone cells. Osteoporos Int 13:137–146
Manolagas SC (1999) Cell number versus cell vigor—what really matters to a regenerating skeleton? Endocrinology 140:4377–4381
Suda T, Miyama K, Uchiyama Y, Katagiri T, Yamaguchi A, Sato T (1994) Osteoporotic bone changes in SAMP6 are due to a decrease in osteoblast progenitor cells. In: Takeda T (ed) The SAM model of senescence: proceedings of the First International Conference on Senescence, the SAM model. Elsevier Science, Kyoto, pp 47–52
Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC (1996) Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest 97:1732–1740
Silva MJ, Brodt MB, Ettner SL (2002) Long bones from the senescence accelerated mouse SAMP6 have increased size but reduced whole-bone strength and resistance to fracture. J Bone Miner Res 17:1597–1603
Silva MJ, Brodt MD, Ko M, Abu-Amer Y (2005) Impaired marrow osteogenesis is associated with reduced endocortical bone formation but does not impair periosteal bone formation in long bones of SAMP6 mice. J Bone Miner Res 20:419–427
Silva MJ, Brodt MD, Wopenka B, Thomopoulos S, Williams D, Wassen MH, Ko M, Kusano N, Bank RA (2006) Decreased collagen organization and content are associated with reduced strength of demineralized and intact bone in the SAMP6 mouse. J Bone Miner Res 21:78–88
Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR (1998) Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int 63:442–449
Silva MJ, Brodt MD, Hucker WJ (2005) Finite element analysis of the mouse tibia: estimating endocortical strain during three-point bending in SAMP6 osteoporotic mice. Anat Rec A Discov Mol Cell Evol Biol 283A:380–390
Grimston SK, Brodt MD, Silva MJ, Civitelli R (2008) Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1). J Bone Miner Res [Epub ahead of print]
Dimai HP, Linkhart TA, Linkhart SG, Donahue LR, Beamer WG, Rosen CJ, Farley JR, Baylink DJ (1998) Alkaline phosphatase levels and osteoprogenitor cell numbers suggest bone formation may contribute to peak bone density differences between two inbred strains of mice. Bone 22:211–216
Aubin JE (1999) Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell–cell interactions in osteoblast differentiation. J Cell Biochem 72:396–410
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Miner Res 2:595–610
Foldes J, Shih MS, Parfitt AM (1990) Frequency distributions of tetracycline-based measurements: implications for the interpretation of bone formation indices in the absence of double-labeled surfaces. J Bone Miner Res 5:1063–1067
Turner CH, Forwood MR, Rho JY, Yoshikawa T (1994) Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res 9:87–97
Nakanishi R, Shimizu M, Mori M, Akiyama H, Okudaira S, Otsuki B, Hashimoto M, Higuchi K, Hosokawa M, Tsuboyama T, Nakamura T (2006) Secreted frizzled-related protein 4 is a negative regulator of peak BMD in SAMP6 mice. J Bone Miner Res 21:1713–1721
Kodama Y, Takeuchi Y, Suzawa M, Fukumoto S, Murayama H, Yamato H, Fujita T, Kurokawa T, Matsumoto T (1998) Reduced expression of interleukin-11 in bone marrow stromal cells of senescence-accelerated mice (SAMP6): relationship to osteopenia with enhanced adipogenesis. J Bone Miner Res 13:1370–1377
Tohjima E, Inoue D, Yamamoto N, Kido S, Ito Y, Kato S, Takeuchi Y, Fukumoto S, Matsumoto T (2003) Decreased AP-1 activity and interleukin-11 expression by bone marrow stromal cells may be associated with impaired bone formation in aged mice. J Bone Miner Res 18:1461–1470
Elias JA, Tang W, Horowitz MC (1995) Cytokine and hormonal stimulation of human osteosarcoma interleukin-11 production. Endocrinology 136:489–498
Kim GS, Kim CH, Choi CS, Park JY, Lee KU (1997) Involvement of different second messengers in parathyroid hormone- and interleukin-1-induced interleukin-6 and interleukin-11 production in human bone marrow stromal cells. J Bone Miner Res 12:896–902
Clement-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssiere B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G (2005) Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci USA 102:17406–17411
Chow JW, Fox S, Jagger CJ, Chambers TJ (1998) Role for parathyroid hormone in mechanical responsiveness of rat bone. Am J Physiol Endocrinol Metab 274:E146–E154
Keila S, Pitaru S, Grosskopf A, Weinreb M (1994) Bone marrow from mechanically unloaded rat bones expresses reduced osteogenic capacity in vitro. J Bone Miner Res 9:321–327
Kostenuik PJ, Halloran BP, Morey-Holton ER, Bikle DD (1997) Skeletal unloading inhibits the in vitro proliferation and differentiation of rat osteoprogenitor cells. Am J Physiol Endocrinol Metab 273:E1133–E1139
Kostenuik PJ, Harris J, Halloran B, Turner RT, Morey-Holton ER, Bikle DD (1999) Skeletal unloading causes resistance of osteoprogenitor cells to parathyroid hormone and to insulin-like growth factor-I. J Bone Miner Res 14:21–31
Chow JW, Wilson AJ, Chambers TJ, Fox SW (1998) Mechanical loading stimulates bone formation by reactivation of bone lining cells in 13-week-old rats. J Bone Miner Res 13:1760–1767
Turner CH, Owan I, Alvey T, Hulman J, Hock JM (1998) Recruitment and proliferative responses of osteoblasts after mechanical loading in vivo determined using sustained-release bromodeoxyuridine. Bone 22:463–469
Akhter MP, Cullen DM, Recker RR (2002) Bone adaptation response to sham and bending stimuli in mice. J Clin Densitom 5:207–216
Acknowledgements
The authors thank Mike Ko for performing the cell culture assays. This study was funded by a grant from the National Institutes of Health (NIAMS AR47867). Animals were housed in a facility supported by a National Institutes of Health grant (NCRR C06 RR015502).
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 2004 meeting of the American Society of Bone and Mineral Research and the 2005 meeting of the Orthopaedic Research Society.
Rights and permissions
About this article
Cite this article
Silva, M.J., Brodt, M.D. Mechanical Stimulation of Bone Formation is Normal in the SAMP6 Mouse. Calcif Tissue Int 82, 489–497 (2008). https://doi.org/10.1007/s00223-008-9142-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-008-9142-5