Abstract
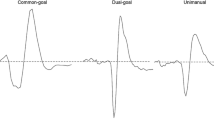
Bimanual coordination is essential for the performance of many everyday tasks. There are several types of bimanually coordinated movements, classified according to whether the arms are acting to achieve a single goal (cooperative) or separate goals (independent), and whether the arms are moving symmetrically or asymmetrically. Symmetric bimanual movements are thought to facilitate corticomotor excitability (CME), while asymmetric bimanual movements are thought to recruit interhemispheric inhibition to reduce functional coupling between the motor cortices. The influences of movement symmetry and goal conceptualisation on interhemispheric interactions have not been studied together, and not during bimanually active dynamic tasks. The present study used transcranial magnetic stimulation (TMS) to investigate the modulation of CME and short- and long-latency interhemispheric inhibition (SIHI and LIHI, respectively) during bimanually active dynamic tasks requiring different types of bimanual coordination. Twenty healthy right-handed adults performed four bimanual tasks in which they held a dumbbell in each hand (independent) or a custom device between both hands (cooperative) while rhythmically flexing and extending their wrists symmetrically or asymmetrically. Motor-evoked potentials were recorded from the right extensor carpi ulnaris. We found CME was greater during asymmetric tasks than symmetric tasks, and movement symmetry did not modulate SIHI or LIHI. There was no effect of goal conceptualisation nor any interaction with movement symmetry for CME, SIHI or LIHI. Based on these results, movement symmetry and goal conceptualisation may not modulate interhemispheric inhibition during dynamic bimanual tasks. These findings contradict prevailing thinking about the roles of CME and interhemispheric inhibition in bimanual coordination.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Arai N, Lu MK, Ugawa Y, Ziemann U (2012) Effective connectivity between human supplementary motor area and primary motor cortex: a paired-coil TMS study. Exp Brain Res 220:79–87. https://doi.org/10.1007/s00221-012-3117-5
Aramaki Y, Osu R, Sadato N (2010) Resource-demanding versus cost-effective bimanual interaction in the brain. Exp Brain Res 203:407–418. https://doi.org/10.1007/s00221-010-2244-0
Awiszus F, Borckdardt JJ (2011) TMS motor threshold assessment tool (MTAT 2.0)
Bailey RR, Klaesner JW, Lang CE (2015) Quantifying real-world upper-limb activity in nondisabled adults and adults with chronic stroke. Neurorehabil Neural Repair 29:969–978. https://doi.org/10.1177/1545968315583720
Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M (2008) Callosal contributions to simultaneous bimanual finger movements. J Neurosci 28:3227–3233. https://doi.org/10.1523/JNEUROSCI.4076-07.2008
Byblow WD, Stinear CM, Smith MC, Bjerre L, Flaskager BK, McCambridge AB (2012) Mirror symmetric bimanual movement priming can increase corticomotor excitability and enhance motor learning. PLoS ONE 7:e33882. https://doi.org/10.1371/journal.pone.0033882PONE-D-11-10075[pii]
Calvert GHM, McMackin R, Carson RG (2020) Probing interhemispheric dorsal premotor-primary motor cortex interactions with threshold hunting transcranial magnetic stimulation. Clin Neurophysiol 131:2551–2560. https://doi.org/10.1016/j.clinph.2020.07.020
Carson RG (2020) Inter-hemispheric inhibition sculpts the output of neural circuits by co-opting the two cerebral hemispheres. J Physiol. https://doi.org/10.1113/JP279793
Carson RG, Riek S, Bawa P (1999) Electromyographic activity, H-reflex modulation and corticospinal input to forearm motoneurones during active and passive rhythmic movements. Hum Mov Sci 18:307–343. https://doi.org/10.1016/S0167-9457(99)00013-5
Chiou SY, Wang RY, Liao KK, Wu YT, Lu CF, Yang YR (2013) Co-activation of primary motor cortex ipsilateral to muscles contracting in a unilateral motor task. Clin Neurophysiol 124:1353–1363. https://doi.org/10.1016/j.clinph.2013.02.001
Cunningham DA et al (2017) The effect of motor overflow on bimanual asymmetric force coordination. Exp Brain Res 235:1097–1105. https://doi.org/10.1007/s00221-016-4867-2
Davidson AG, Buford JA (2006) Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173:25–39. https://doi.org/10.1007/s00221-006-0374-1
Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2004) Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 21:1416–1427. https://doi.org/10.1016/j.neuroimage.2003.12.011
Diedrichsen J (2007) Optimal task-dependent changes of bimanual feedback control and adaptation. Current Biol 17:1675–1679. https://doi.org/10.1016/j.cub.2007.08.051
Dietz V, Macauda G, Schrafl-Altermatt M, Wirz M, Kloter E, Michels L (2015) Neural coupling of cooperative hand movements: a reflex and fMRI study. Cereb Cortex 25:948–958. https://doi.org/10.1093/cercor/bht285
Fujiyama H, Van Soom J, Rens G, Gooijers J, Leunissen I, Levin O, Swinnen SP (2016) Age-related changes in frontal network structural and functional connectivity in relation to bimanual movement control. J Neurosci 36:1808–1822. https://doi.org/10.1523/Jneurosci.3355-15.2016
Green PE, Ridding MC, Hill KD, Semmler JG, Drummond PD, Vallence AM (2018) Supplementary motor area-primary motor cortex facilitation in younger but not older adults. Neurobiol Aging 64:85–91. https://doi.org/10.1016/j.neurobiolaging.2017.12.016
Hinder MR, Puri R, Kemp S, Waitzer S, Reissig P, Stockel T, Fujiyama H (2018) Distinct modulation of interhemispheric inhibitory mechanisms during movement preparation reveals the influence of cognition on action control. Cortex 99:13–29. https://doi.org/10.1016/j.cortex.2017.10.002
Hiraoka K et al (2014) Bimanual coordination of force enhances interhemispheric inhibition between the primary motor cortices. NeuroReport 25:1203–1207. https://doi.org/10.1097/WNR.0000000000000248
Hoyer EH, Bastian AJ (2013) The effects of task demands on bimanual skill acquisition. Exp Brain Res 226:193–208. https://doi.org/10.1007/s00221-013-3425-4
Irlbacher K, Brocke J, Mechow JV, Brandt SA (2007) Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clin Neurophysiol 118:308–316. https://doi.org/10.1016/j.clinph.2006.09.023
Kantak S, Jax S, Wittenberg G (2017) Bimanual coordination: a missing piece of arm rehabilitation after stroke. Restor Neurol Neurosci 35:347–364. https://doi.org/10.3233/RNN-170737
Kantak S, McGrath R, Zahedi N (2016) Goal conceptualization and symmetry of arm movements affect bimanual coordination in individuals after stroke. Neurosci Lett 626:86–93. https://doi.org/10.1016/j.neulet.2016.04.064
Kennerley SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB (2002) Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nat Neurosci 5:376–381. https://doi.org/10.1038/nn822
Khong KYW, Galan F, Soteropoulos DS (2020) Rapid crossed responses in an intrinsic hand muscle during perturbed bimanual movements. J Neurophysiol 123:630–644. https://doi.org/10.1152/jn.00282.2019
Kim RK, Kang N (2020) Bimanual coordination functions between paretic and nonparetic arms: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 29:104544. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104544
Kraft E, Chen AW, Flaherty AW, Blood AJ, Kwong KK, Jenkins BG (2007) The role of the basal ganglia in bimanual coordination. Brain Res 1151:62–73. https://doi.org/10.1016/j.brainres.2007.01.142
Liuzzi G, Horniss V, Zimerman M, Gerloff C, Hummel FC (2011) Coordination of uncoupled bimanual movements by strictly timed interhemispheric connectivity. J Neurosci 31:9111–9117. https://doi.org/10.1523/JNEUROSCI.0046-11.2011
Matsuda T, Watanabe S, Kuruma H, Murakami Y, Watanabe R, Senou A (2009) A comparison of three bimanual coordinations: an fMRI study. J Phys Ther Sci 21:85–92. https://doi.org/10.1589/jpts.21.85
Mooney RA, Cirillo J, Byblow WD (2018) Adaptive threshold hunting reveals differences in interhemispheric inhibition between young and older adults. Eur J Neurosci 48:2247–2258. https://doi.org/10.1111/ejn.14097
Morishita T, Kubota S, Hirano M, Funase K (2014) Different modulation of short and long-latency interhemispheric inhibition from active to resting primary motor cortex during a fine-motor manipulation task. Physiol Rep https://doi.org/10.14814/phy2.12170
Morishita T, Uehara K, Funase K (2012) Changes in interhemispheric inhibition from active to resting primary motor cortex during a fine-motor manipulation task. J Neurophysiol 107:3086–3094. https://doi.org/10.1152/jn.00888.2011
Mutha PK, Sainburg RL (2009) Shared bimanual tasks elicit bimanual reflexes during movement. J Neurophysiol 102:3142–3155. https://doi.org/10.1152/jn.91335.2008
Nelson AJ, Hoque T, Gunraj C, Ni Z, Chen R (2009) Bi-directional interhemispheric inhibition during unimanual sustained contractions. Bmc Neurosci. https://doi.org/10.1186/1471-2202-10-31
Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, Chen R (2009) Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex 19:1654–1665. https://doi.org/10.1093/cercor/bhn201bhn201[pii]
Nomura Y, Jono Y, Tani K, Chujo Y, Hiraoka K (2016) Corticospinal modulations during bimanual movement with different relative phases. Front Hum Neurosci 10:95. https://doi.org/10.3389/fnhum.2016.00095
Oliveira FT, Ivry RB (2008) The representation of action: insights from bimanual coordination. Curr Dir Psychol Sci 17:130–135. https://doi.org/10.1111/j.1467-8721.2008.00562.x
Patel P, Lodha N (2020) Functional implications of impaired bimanual force coordination in chronic stroke. Neurosci Lett 738:135387. https://doi.org/10.1016/j.neulet.2020.135387
Perez MA, Butler JE, Taylor JL (2014) Modulation of transcallosal inhibition by bilateral activation of agonist and antagonist proximal arm muscles. J Neurophysiol 111:405–414. https://doi.org/10.1152/jn.00322.2013
Rom DM (1990) A sequentially rejective test procedure based on a modified Bonferroni inequality. Biometrika 77:663–665
Ruddy KL, Carson RG (2013) Neural pathways mediating cross education of motor function. Front Hum Neurosci 7:397. https://doi.org/10.3389/fnhum.2013.00397
Ruddy KL, Leemans A, Carson RG (2017) Transcallosal connectivity of the human cortical motor network. Brain Struct Funct 222:1243–1252. https://doi.org/10.1007/s00429-016-1274-1
Sattler V, Dickler M, Michaud M, Simonetta-Moreau M (2012) Interhemispheric inhibition in human wrist muscles. Exp Brain Res 221:449–458. https://doi.org/10.1007/s00221-012-3187-4
Schrafl-Altermatt M, Dietz V (2016) Cooperative hand movements in post-stroke subjects: neural reorganization. Clin Neurophysiol 127:748–754. https://doi.org/10.1016/j.clinph.2015.07.004
Serrien DJ, Nirkko AC, Wiesendanger M (2001) Role of the corpus callosum in bimanual coordination: a comparison of patients with congenital and acquired callosal damage. Eur J Neurosci 14:1897–1905. https://doi.org/10.1046/j.0953-816x.2001.01798.x
Soteropoulos DS, Perez MA (2011) Physiological changes underlying bilateral isometric arm voluntary contractions in healthy humans. J Neurophysiol 105:1594–1602. https://doi.org/10.1152/jn.00678.2010
Stinear JW, Byblow WD (2002) Disinhibition in the human motor cortex is enhanced by synchronous upper limb movements. J Physiol 543:307–316
Stinear JW, Byblow WD (2004) An interhemispheric asymmetry in motor cortex disinhibition during bimanual movement. Brain Res 1022:81–87. https://doi.org/10.1016/j.brainres.2004.06.062S0006-8993(04)01076-5[pii]
Swinnen SP (2002) Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci 3:348–359. https://doi.org/10.1038/nrn807
Tazoe T, Komiyama T (2014) Interlimb neural interactions in the corticospinal pathways. J Sports Med Phys Fitness 3:181–190
Turco CV, Fassett HJ, Locke MB, El-Sayes J, Nelson AJ (2019) Parallel modulation of interhemispheric inhibition and the size of a cortical hand muscle representation during active contraction. J Neurophysiol 122:368–377. https://doi.org/10.1152/jn.00030.2019
Veale JF (2014) Edinburgh handedness inventory—short form: a revised version based on confirmatory factor analysis. Laterality 19:164–177. https://doi.org/10.1080/1357650X.2013.783045
Wiegel P, Kurz A, Leukel C (2020) Evidence that distinct human primary motor cortex circuits control discrete and rhythmic movements. J Physiol-London 598:1235–1251. https://doi.org/10.1113/Jp278779
Wu T, Wang L, Hallett M, Li K, Chan P (2010) Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain 133:2394–2409. https://doi.org/10.1093/brain/awq151
Yedimenko JA, Perez MA (2010) The effect of bilateral isometric forces in different directions on motor cortical function in humans. J Neurophysiol 104:2922–2931. https://doi.org/10.1152/jn.00020.2010
Zehr EP, Collins DF, Frigon A, Hoogenboom N (2003) Neural control of rhythmic human arm movement: phase dependence and task modulation of hoffmann reflexes in forearm muscles. J Neurophysiol 89:12–21. https://doi.org/10.1152/jn.00416.2002
Acknowledgements
We thank Kelly Ho, Felix Thomas, and April Ren for their assistance with data collection.
Funding
There is no funding to disclose.
Author information
Authors and Affiliations
Contributions
All authors helped with designing the experiment. HJ and MSA carried out data collection. HJ and CS performed the statistical analysis. HJ wrote the manuscript, which was revised by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University of Auckland (approved on 30/10/18, Reference Number 022023).
Informed consent
Participants gave their written informed consent and this study was approved by the institutional ethics committee.
Consent for publication
Participants signed informed consent regarding publishing their data.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jordan, H.T., Schrafl-Altermatt, M., Byblow, W.D. et al. The modulation of short and long-latency interhemispheric inhibition during bimanually coordinated movements. Exp Brain Res 239, 1507–1516 (2021). https://doi.org/10.1007/s00221-021-06074-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06074-z