Abstract
Reaching to a veridical target permits an egocentric spatial code (i.e., absolute limb and target position) to effect fast and effective online trajectory corrections supported via the visuomotor networks of the dorsal visual pathway. In contrast, a response entailing decoupled spatial relations between stimulus and response is thought to be primarily mediated via an allocentric code (i.e., the position of a target relative to another external cue) laid down by the visuoperceptual networks of the ventral visual pathway. Because the ventral stream renders a temporally durable percept, it is thought that an allocentric code does not support a primarily online mode of control, but instead supports a mode wherein a response is evoked largely in advance of movement onset via central planning mechanisms (i.e., offline control). Here, we examined whether reaches defined via ego- and allocentric visual coordinates are supported via distinct control modes (i.e., online versus offline). Participants performed target-directed and allocentric reaches in limb visible and limb-occluded conditions. Notably, in the allocentric task, participants reached to a location that matched the position of a target stimulus relative to a reference stimulus, and to examine online trajectory amendments, we computed the proportion of variance explained (i.e., R2 values) by the spatial position of the limb at 75% of movement time relative to a response’s ultimate movement endpoint. Target-directed trials performed with limb vision showed more online corrections and greater endpoint precision than their limb-occluded counterparts, which in turn were associated with performance metrics comparable to allocentric trials performed with and without limb vision. Accordingly, we propose that the absence of ego-motion cues (i.e., limb vision) and/or the specification of a response via an allocentric code renders motor output served via the ‘slow’ visuoperceptual networks of the ventral visual pathway.






Similar content being viewed by others
Notes
Thaler and Goodale (2011a, b, c) and the current study employed a point representation of the limb (i.e., a computer cursor or LED) and could therefore provide reduced external validity compared to a reaching response involving veridical limb vision. Notably, however, it has been shown that reaches with a point representation provide equivalent online control characteristics to their veridical counterparts (Heath 2005; Heath et al. 2004). What is more, human–machine/computer interactions involve a point representation. Thus, a point representation mirrors the environment associated with many of our day-to-day leisure and occupational actions.
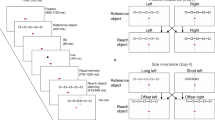
The visual stimuli used here were designed to closely match Thaler and Goodale (2011a) (see Fig. 1 of that experiment); however, the target amplitudes differed between experiments. Specifically, the resultant target amplitudes used in Thaler and Goodale were 122 and 152 mm compared to the 257 and 283 mm amplitudes used here. The longer amplitudes are based on work demonstrating that increasing target amplitude in peripersonal space increases the reliance on feedback-based trajectory amendments (Elliott et al. 1999; Heath 2005; Heath et al. 2004; Lemay and Proteau 2001).
Target vectors were computed on a trial-by-trial basis. For the target-directed condition the magnitude (i.e. distance) and orientation (i.e. direction) of a vector connecting the participant’s start position and the target’s end position was calculated. For the allocentric condition the magnitude and orientation of the vector connecting the reference circle to the target circle was calculated and superimposed onto the participant’s start position.
Our manipulation of target amplitude did not differentially influence target-directed or allocentric tasks. For that reason, target amplitude served as a collapsed factor in our ANOVA model.
References
Binsted G, Heath M (2004) Can the motor system utilize a stored representation to control movement? Behav Brain Sci 27:25–27
Brainard DH (1997) The psychophysics toolbox. Spat Vis 10:433–436
Bridgeman B, Lewis S, Heit G, Nagle M (1979) Relation between cognitive and motor-oriented systems of visual position perception. J Exp Psychol Hum Percept Perform 5:692
Bryden MP (1977) Measuring handedness with questionnaires. Neuropsychologia 15:617–624
Day BL, Lyon IN (2000) Voluntary modification of automatic arm movements evoked by motion of a visual target. Exp Brain Res 130:159–168
Desmurget M, Rossetti Y, Prablanc C, Jeannerod M, Stelmach GE (1995) Representation of hand position prior to movement and motor variability. Can J Physiol Pharmacol 73:262–272
Elliott D, Madalena J (1987) The influence of premovement visual information on manual aiming. Q J Exp Psychol A 39:541–559
Elliott D, Binsted G, Heath M (1999) The control of goal-directed limb movements: correcting errors in the trajectory. Hum Mov Sci 18:121–136
Elliott D, Helsen WF, Chua R (2001) A century later: Woodworth’s (1899) two-component model of goal-directed aiming. Psychol Bull 127:342–357
Elliott D, Hansen S, Grierson LE, Lyons J, Bennett SJ, Hayes SJ (2010) Goal-directed aiming: two components but multiple processes. Psychol Bull 136:1023
Fitts PM (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47:381
Georgopoulos AP (1991) Higher order motor control. Annu Rev Neurosci 14:361–377
Goodale MA (2014) How (and why) the visual control of action differs from visual perception. Proc Biol Sci 281:20140337
Goodale MA, Westwood DA (2004) An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol 14:203–211
Goodale MA, Pelisson D, Prablanc C (1986) Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature 320:748–750
Gréa H, Pisella L, Rossetti Y, Desmurget M, Tilikete C, Grafton S, Prablanc C, Vighetto A (2002) A lesion of the posterior parietal cortex disrupts on-line adjustments during aiming movements. Neuropsychologia 40:2471–2480
Heath M (2005) Role of limb and target vision in the online control of memory-guided reaches. Mot Control 9:281–309
Heath M, Westwood DA (2003) Can a visual representation support the online control of memory-dependent reaching? Evidence from a variable spatial mapping paradigm. Mot Control 7:349–365
Heath M, Westwood DA, Binsted G (2004) The control of memory-guided reaching movements in peripersonal space. Mot Control 8:76–106
Heath M, Maraj A, Gradkowski A, Binsted G (2009) Anti-pointing is mediated by a perceptual bias of target location in left and right visual space. Exp Brain Res 192:275–286
Heath M, Dunham K, Binsted G, Godbolt B (2010a) Antisaccades exhibit diminished online control relative to prosaccades. Exp Brain Res 203:743–752
Heath M, Neely K, Krigolson O, Binsted G (2010b) Memory-guided reaching: what the visuomotor system knows and how long it knows it. In: Elliott D, Khan M (eds) Vision and movement: control of goal-directed action. Human Kinetics, Champaign, IL, pp 79–96
James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA (2002) Differential effects of viewpoint on object-driven activation in dorsal and ventral streams. Neuron 35:793–801
James TW, Culham J, Humphrey GK, Milner AD, Goodale MA (2003) Ventral occipital lesions impair object recognition but not object-directed grasping: an fMRI study. Brain 126:2463–2475
Johnson H, Van Beers RJ, Haggard P (2002) Action and awareness in pointing tasks. Exp Brain Res 146:451–459
Lemay M, Proteau L (2001) A distance effect in a manual aiming task to remembered targets: a test of three hypotheses. Exp Brain Res 140:357–368
Loftus GR, Masson ME (1994) Using confidence intervals in within-subject designs. Psychon Bull Rev 1:476–490
Maraj A, Heath M (2010) Antipointing: perception-based visual information renders an offline mode of control. Exp Brain Res 202:55–64
Messier J, Kalaska JF (1999) Comparison of variability of initial kinematics and endpoints of reaching movements. Exp Brain Res 125:139–152
Neely KA, Heath M, Binsted G (2008) Egocentric and allocentric visual cues influence the specification of movement distance and direction. J Mot Behav 40:203–213
Pisella L, Gréa H, Tilikete C, Vighetto A, Desmurget M, Rode G, Boisson D, Rossetti Y (2000) An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci 3:729–736
Prablanc C, Echallier JE, Jeannerod M, Komilis E (1979) Optimal response of eye and hand motor systems in pointing at a visual target. II. Static and dynamic visual cues in the control of hand movement. Biol Cybern 35:183–187
Rossetti Y, Revol P, McIntosh R, Pisella L, Rode G, Danckert J, Tilikete C, Dijkerman HC, Boisson D, Vighetto A, Michel F, Milner AD (2005) Visually guided reaching: bilateral posterior parietal lesions cause a switch from fast visuomotor to slow cognitive control. Neuropsychologia 43:162–177
Rossit S, Malhotra P, Muir K, Reeves I, Duncan G, Harvey M (2011) The role of right temporal lobe structures in off-line action: evidence from lesion-behavior mapping in stroke patients. Cereb Cortex 21:2751–2761
Schenk T (2006) An allocentric rather than perceptual deficit in patient DF. Nat Neurosci 9:1369–1370
Schmidt RA, Zelaznik H, Hawkins B, Frank JS, Quinn JT Jr (1979) Motor-output variability: a theory for the accuracy of rapid motor acts. Psychol Rev 86:415
Thaler L, Goodale MA (2011a) The role of online visual feedback for the control of target-directed and allocentric hand movements. J Neurophysiol 105:846–859
Thaler L, Goodale MA (2011b) Neural substrates of visual spatial coding and visual feedback control for hand movements in allocentric and target-directed tasks. Front Hum Neurosci 5:92
Thaler L, Goodale MA (2011c) Reaction times for allocentric movements are 35 ms slower than reaction times for target-directed movements. Exp Brain Res 211:313–328
van Donkelaar P, Lee RG, Gellman RS (1994) The contribution of retinal and extraretinal signals to manual tracking movements. Exp Brain Res 99:155–163
Westwood DA, Goodale MA (2003) Perceptual illusion and the real-time control of action. Spat Vis 16:243–254
Acknowledgements
Supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada and Faculty Scholar and Major Academic Development Fund Awards from the University of Western Ontario.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interests involved.
Rights and permissions
About this article
Cite this article
Manzone, J., Heath, M. Goal-directed reaching: the allocentric coding of target location renders an offline mode of control. Exp Brain Res 236, 1149–1159 (2018). https://doi.org/10.1007/s00221-018-5205-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5205-7