Abstract
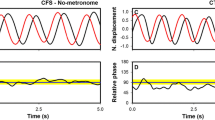
The influence of monitoring-pressure on the performance of anti-phase and in-phase bimanual coordination was examined. The two bimanual patterns were produced under no-monitoring and monitoring-pressure conditions at self-paced frequencies. Anti-phase coordination was always less stable than in-phase coordination, with or without monitoring. When performed under monitoring-pressure, the coordination patterns were performed with less variability in relative phase for both patterns across a range of self-paced movement frequencies compared to performance without monitoring. Thus, while monitoring-pressure did induce a behavioral change, it consisted of performance stabilization rather than degradation, a finding inconsistent with explicit-monitoring theory. However, the findings are consistent with the theory of coordination dynamics and studies that have revealed increased stability for the system’s intrinsic dynamics as a result of attentional focus and intentional control.


Similar content being viewed by others
Notes
The feedback telling participants that their performance was “not good” may be viewed as a form of negative feedback. However, participants were not informed that “not good” meant large error, moving too slow, producing too large a movement, etc. Thus, the information was not directed at a specific aspect of the coordination pattern to change or alter. The information was given within the context of monitoring pressure, i.e., this is why you must be monitored by an expert, and when taken together, the instructions were directed at increasing pressure around being monitored by an expert.
A reviewer suggested that an ANCOVA (analysis of covariance) with movement frequency as a covariate would be another way to analysis the relationship between movement frequency and standard deviation instead of using the MVF ratio. An ANCOVA of the relative phase variability data with movement frequency as a covariate and Monitoring and Pattern as factors produced the following results. Movement frequency was a significant covariate, F (1, 19) = 33.5, p < .0001, \({\eta }_{p}^{2}\)=.064. The ANCOVA also found a significant effect of Pattern, F (1, 19) \({\eta }_{p}^{2}\) = 11.2, p < 0.01, = 0.29, and a significant effect of Monitoring, F (1, 19) = 7.9, p = 0.011, \({\eta }_{p}^{2}\) = 0.29. Relative phase variability was larger for the anti-phase (Mn = 20°) compared to the in-phase (Mn = 15°) pattern in block 3. This Pattern effect is consistent with the analysis of the variability data without movement frequency as a covariate. The ANCOVA monitoring effect revealed that variability was smaller under the monitoring-pressure (Mn = 16°) compared to the no-monitoring pressure condition (Mn = 20°). This monitoring effect was not significant in the ANOVA analysis of the block 3 relative phase variability data, however, the monitoring effect was significant in the ANOVA analysis of the MVF ratio. These results show that movement frequency did contribute to relative phase variability in a significant way in this experiment. This is an expected finding, since extensive research has shown these bimanual patterns, especially anti-phase, to be sensitive to movement frequency changes when explicitly controlled with pacing signals.
References
Abrahamse EL, Ruitenberg MFL, de Kleine E, Verwey WB (2013) Control of automated behavior: insights from the discrete sequence production task. Front Hum Neurosci 7:82. doi:10.3389/fnhum.2013.00082
Baumeister RF (1984) Choking under pressure—self-consciousness and paradoxical effects of incentives on skillful performance. J Pers Soc Psychol 46:610–620
Beilock SL, Carr TH (2001) On the fragility of skilled performance: what governs choking under pressure? J Exp Psychol Gen 130:701–725
Beilock SL, DeCaro MS (2007) From poor performance to success under stress: working memory, strategy selection, and mathematical problem solving under pressure. J Exp Psychol Learn Mem Cogn 33:983–998
Buchanan JJ (2013) Flexibility in the control of rapid aiming actions. Exp Brain Res 229:47–60. doi:10.1007/s00221-013-3589-y
Buchanan JJ (2015) Perceptual estimates of motor skill proficiency are constrained by the stability of coordination patterns. J Mot Behav 47:453–464. doi:10.1080/00222895.2015.1008687
Buchanan JJ, Ryu YU (2012) Scaling movement amplitude: adaptation of timing and amplitude control in a bimanual task. J Mot Behav 44:135–147. doi:10.1080/00222895.2012.656158
Buchanan JJ, Wang CY (2012) Overcoming the guidance effect in motor skill learning: feedback all the time can be beneficial. Exp Brain Res 219:305–320. doi:10.1007/s00221-012-3092-x
Buchanan JJ, Kelso JAS, deGuzman GD, Ding M (1997) The spontaneous recruitment and suppression of degrees of freedom in rhythmic hand movements. Hum Mov Sci 16:1–32
Buchanan JJ, Ramos J, Robson N (2015) The perception–action dynamics of action competency are altered by both physical and observational training. Exp Brain Res 233:1289–1305. doi:10.1007/s00221-015-4207-y
Byblow WD, Carson RG, Goodman D (1994) Expressions of asymmetries and anchoring in bimanual coordination. Hum Mov Sci 13:3–28
Dayan E, Cohen LG (2011) Neuroplasticity subserving motor skill learning. Neuron 72:443–454. doi: 10.1016/j.neuron.2011.10.008
DeCaro MS, Thomas RD, Albert NB, Beilock SL (2011) Choking under pressure: multiple routes to skill failure. J Exp Psychol Gen 140:390–406
Doyon J, Bellec P, Amsel R et al (2009) Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199:61–75. doi:10.1016/j.bbr.2008.11.012
Fink PW, Foo P, Jirsa VK, Kelso JAS (2000) Local and global stabilization of coordination by sensory information. Exp Brain Res 134:12
Forrester L, Whitall J (2000) Bimanual finger tapping: effects of frequency and auditory information on timing consistency and coordination. J Mot Behav 32:176–191
Gray R (2004) Skilled performance in baseball batting: understanding successes and failures. J Sport Exerc Psychol 26:S82–S82
Guiard Y (1993) On Fitts’s and Hooke’s laws: simple harmonic movement in upper-limb cyclical aiming. Acta Psychol 82:139–159
Haken H, Kelso JAS, Bunz H (1985) A theoretical model of phase transitions in human hand movements. Biol Cybern 51:347–356
Hardwick RM, Rottschy C, Miall RC, Eickhoff SB (2013) A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67:283–297
Hessler EE, Gonzales LM, Amazeen PG (2010) Displays that facilitate performance of multifrequency ratios during motor-respiratory coordination. Acta Psychol 133:96
Hiraga CY, Summers JJ, Temprado JJ (2004) Attentional costs of coordinating homologous and non-homologous limbs. Hum Mov Sci 23:415–430. doi:10.1016/j.humov.2004.08.015
Hiraga CY, Summers JJ, Temprado JJ (2005) Effects of attentional prioritisation on the temporal and spatial components of an interlimb circle-drawing task. Hum Mov Sci 24:815–832. doi:10.1016/j.humov.2005.10.010
Kelso JAS (1984) Phase transitions and critical behavior in human bimanual coordination. Am J Physiol 246:1000–1004
Kelso JAS (1994) The informational character of self-organized coordination dynamics. Hum Mov Sci 13:393–413
Kelso JAS, Scholz JP, Schoner G (1986) Nonequilibrium phase-transitions in coordinated biological motion—critical fluctuations. Phys Lett A 118:279–284
Kovacs AJ, Buchanan JJ, Shea CH (2009) Bimanual 1:1 with 90° degrees continuous relative phase: difficult or easy! Exp Brain Res 193:129–136. doi:10.1007/s00221-008-1676-2
Lee TD, Swinnen SP, Verschueren S (1995) Relative phase alterations during bimanual skill acquisition. J Mot Behav 27:263–274
Lewis BP, Linder DE (1997) Thinking about choking? Attentional processes and paradoxical performance. Pers Soc Psychol Bull 23:937–944
Markman AB, Maddox WT, Worthy DA (2006) Choking and excelling under pressure. Psychol Sci 17:944–948
Masters RSW (1992) Knowledge, knerves and know-how—the Role of explicit versus implicit knowledge in the breakdown of a complex motor skill under pressure. Br J Psychol 83:343–358
Monno A, Chardenon A, Temprado JJ, Zanone PG, Laurent M (2000) Effects of attention on phase transitions between bimanual coordination patterns: a behavioral and cost analysis in humans. Neurosci Lett 283:93–96. doi:10.1016/s0304-3940(00)00924-1
Monno A, Temprado JJ, Zanone PG, Laurent M (2002) The interplay of attention and bimanual coordination dynamics. Acta Psychol 110:187–211. doi:10.1016/s0001-6918(02)00033-1
Nissen MJ, Bullemer P (1987) Attentional requirements of learning—evidence from performance−Measures. Cogn Psychol 19:1–32
Rhodes BJ, Bullock D, Verwey WB, Averbeck BB, Page MPA (2004) Learning and production of movement sequences: Behavioral, neurophysiological, and modeling perspectives. Hum Mov Sci 23:699–746
Ryu YU, Buchanan JJ (2004) Amplitude Scaling in a bimanual circle drawing task: pattern switching and end-effector variability. J Mot Behav 36:265–275
Schöner G, Kelso JAS (1988) Dynamic pattern generation in behavioral and neural systems. Science 239:1513–1520
Schöner G, Haken H, Kelso JAS (1986) A stochastic theory of phase transitions in human hand movement. Biol Cybern 53:247–257
Temprado JJ, Zanone PG, Monno A, Laurent M (1999) Attentional load associated with performing and stabilizing preferred bimanual patterns. J Exp Psychol Hum Percept Perform 25:1579–1594
Temprado JJ, Chardenon A, Laurent M (2001a) Interplay of biomechanical and neuromuscular constraints on pattern stability and attentional demands in a bimanual coordination task in human subjects. Neurosci Lett 303:127–131. doi:10.1016/s0304-3940(01)01650-0
Temprado JJ, Zanone PG, Monno A, Laurent M (2001b) A dynamical framework to understand performance trade-offs and interference in dual tasks. J Exp Psychol Hum Percept Perform 27:1303–1313. doi:10.1037//0096-1523.27.6.1303
Tuller B, Kelso JAS (1989) Environmentally-specified patterns of movement coordination in normal and split-brain subjects. Exp Brain Res 75:306–316
Verwey WB, Shea CH, Wright DL (2015) A cognitive framework for explaining serial processing and sequence execution strategies. Psychon Bull Rev 22:54–77
Wilson AD, Collins DR, Bingham GP (2005) Perceptual coupling in rhythmic movement coordination: stable perception leads to stable action. Exp Brain Res 164:517–528
Wine J (1971) Test anxiety and direction of attention. Psychol Bull 76:92–104
Wright D, Verwey W, Buchanan JJ, Chen J, Rhee J, Maarteen I (2016) Consolidating behavioral and neurophysiologic findings to explain the influence of contextual interference during motor sequence learning. Psychon Bull Rev 23:1–21. doi:10.3758/s13423-015-0887-3
Zanone PG, Kelso JAS (1992) The evolution of behavioral attractors with learning: nonequilibrium phase transitions. J Exp Psychol Hum Percept Perform 18:403–421
Zanone PG, Monno A, Temprado JJ, Laurent M (2001) Shared dynamics of attentional cost and pattern stability. Hum Mov Sci 20:765–789. doi:10.1016/s0167-9457(01)00055-0
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buchanan, J.J., Park, I., Chen, J. et al. Bimanual coordination patterns are stabilized under monitoring-pressure. Exp Brain Res 235, 1909–1918 (2017). https://doi.org/10.1007/s00221-016-4869-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4869-0