Abstract
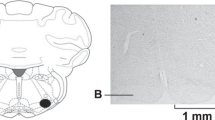
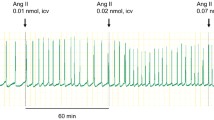
The present study was carried out to clarify the role of noradrenergic systems in the mediation of drinking response to angiotensin II (ANG II) in the rat subfornical organ (SFO). Microinjection of ANG II (10 pmol, 50 nl) into the SFO caused a robust drinking response (water volume, 1.8–8.7 ml for 20 min). Injections of either noradrenaline (NA; 0.1, 1 and 10 nmols, 50 nl) into the SFO did not produce a significant water intake. Phenylephrine (Phen; 0.1, 1, 10 nmols, 50 nl), an α1-adrenoceptor agonist, injected into the SFO elicited little drinking in the rats tested (water volume, 0.4–1.5 ml for 20 min). Previous injections of NA (0.1 and 1 nmols) or Phen (0.1, 1 and 10 nmols) significantly enhanced the water intake elicited by the injection of ANG II into the SFO. Neither the α2-adrenoceptor agonist clonidine (Clon; 10 nmol, 50 nl) nor the β-adrenoceptor agonist isoprenaline (Isop; 10 nmol, 50 nl) into the SFO caused a significant water intake. Previous injections of Clon (0.1, 1 and 10 nmol, 50 nl) into the SFO were without effect on the water intake produced by the ANG II injection into the SFO. Pretreatment with Isop (1 and 10 nmols), on the other hand, significantly attenuated the drinking response to ANG II. Vehicle (artificial cerebrospinal fluid, 50 nl) had no effect on the ANG II-induced water intake. These results suggest that both α1-(facilitatory) and β-(inhibitory) adrenoceptor mechanisms may be implicated in the control of drinking response induced by angiotensinergic activation of SFO neurons.


Similar content being viewed by others
References
Abe M, Tokunaga T, Yamada K, Fukukawa T (1988) Gamma-aminobutyric acid and taurine antagonize the central effects of angiotensin II and renin on the intake of water and salt, and on blood pressure in rats. Neuropharmacology 27:309–318
Almeida NAA, Antunes VR, Saad WA, Camargo LAA (1999) Effects of alpha antagonists and agonists injected into the lateral hypothalamus on water and sodium intake induced by angiotensin II injection into the subfornical organ. Brain Res Bull 48:521–525
Camargo LAA, Saad WA, Camargo GPA (2000) Effects of subtypes alpha- and beta-adrenoceptors of the lateral hypothalamus on the water and sodium intake induced by angiotensin II injected into the subfornical organ. Brain Res 8811:176–181
Ciriello J, Rosa-Arellani P, Solanor-Flores R (1996) Direct projections to subfornical organ from catecholaminergic neurons in the caudal nucleus of the solitary tract. Brain Res 726:227–232
Eng R, Miselis R (1981) Polydipsia and abolition of angiotensin-induced drinking after transection of subfornical organ efferent projections in the rat. Brain Res 255:200–206
Ferguson AV, Kasting NW (1986) Electrical stimulation in the subfornical organ increases plasma vasopressin concentrations in the conscious rat. Am J Physiol 251:R425–R428
Ferguson AV, Renaud LP (1984) Hypothalamic paraventricular nucleus lesions decrease pressor responses to subfornical organ stimulation. Brain Res 305:361–364
Fujisawa S, Tanaka J, Nomura M (2001) Estrogen attenuates the drinking response induced by activation of angiotensinergic pathways from the lateral hypothalamic area to the subfornical organ in female rats. Behav Brain Res 122:33–41
Grossman SP (1984) A reassessment of the brain mechanisms that control thirst. Neurosci Biobehav Rev 6:95–104
Gu G-B, Ju G (1995) The parabrachio-subfornical organ projection in the rat. Brain Res Bull 38:41–47
Gutmann MB, Jones DL, Ciriello J (1988) Effect of paraventricular nucleus lesions on drinking and pressor responses to ANG II. Am J Physiol 255:R882–R887
Inenaga K, Nagamoto T, Honda E, Ueta Y, Yamashita H (1995) GABAergic inhibitory inputs to subfornical organ neurons in rat slice preparations. Brain Res 705:85–90
Iovino M, Steardo M (1984) Vasopressin release to central peripheral angiotensin II in rats with lesions of the subfornical organ. Brain Res 322:365–368
Jones DL, Mogenson GJ (1982) Central injections of spiperone and GABA: attenuation of angiotensin II stimulated thirst. Can J Physiol Pharmacol 60:720–726
Kawano H, Masuko S (2001) Tyrosin hydroxylase-immunoreactive projections from the caudal ventrolateral medulla to the subfornical organ in the rat. Brain Res 903:154–161
Knepel W, Nutto D, Meyer DK (1984) Effect of transection of subfornical organ efferent projections on vasopressin release induced by angiotensin or isoprenaline in the rat. Brain Res 248:180–184
Lind RW, Johnson AK (1982) Subfornical organ-median preoptic connections and drinking and pressor responses to angiotensin II. J Neurosci 2:1043–1051
Lind RW, Swanson LW, Ganten D (1984) Angiotensin II immunoreactivity in neural afferents and efferents of the subfornical organ of the rat. Brain Res 321:209–215
Lind RW, Swanson LW, Ganten D (1985) Organization of angiotensin II immunoreactive cell and fibers in the rat central nervous system: an immunohistochemical study. Neuroendocrinology 40:2–24
Mangiapane ML, Simpson JB (1980) Subfornical organ: forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol 239:R382–R389
Mangiapane ML, Thrasher TN, Keil LC, Simpson JB, Ganong WF (1984) Role of the subfornical organ in vasopressin release. Brain Res Bull 13:74–77
McKinley MJ, McAllen RM, Mendelsohn FAO, Allen AM, Chai SY, Oldfield BJ (1990) Circumventricular organ: neuroendocrine interfaces between the brain and the hemal milieu. Front Neuroendocrinol 11:91–127
McKitrick DJ, Krukoff TL, Calaresu FR (1992) Expression of c-fos protein in rat brain after electrical stimulation of the aortic depressor nerve. Brain Res 599:215–222
Mok D, Mogenson GJ (1987) Convergence of signals in the zona incerta for angiotensin-mediated and osmotic thirst. Brain Res 407:332–340
Osaka T, Yamashita H, Koizumi K (1992) Inhibitory inputs to the subfornical organ from the AV3 V: involvement of GABA. Brain Res Bull 29:581–587
Rosas-Arellano MP, Solano-Flores LP, Ciriello J (1995) Glutamate stimulation of arcuate nucleus inhibits responses of subfornical organ neurons to plasma hypernatremia and angiotensin II. Neurosci Lett 198:201–204
Shioya M, Tanaka J (1989) Inputs from the nucleus of the solitary tract to subfornical organ neurons projecting to the paraventricular nucleus in the rat. Brain Res 483:192–195
Tanaka J, Nomura M (1993) Involvement of neurons sensitive to angiotensin II in the median preoptic nucleus in the drinking response induced by angiotensin II activation of the subfornical organ. Exp Neurol 119:235–239
Tanaka J, Seto K (1988) Neurons in the nucleus of the solitary tract with ascending projections to the subfornical organ in the rat. Neurosci Lett 89:152–155
Tanaka J, Kaba H, Saito H, Seto K (1986) Lateral hypothalamic area stimulation excites neurons in the region of the subfornical organ with efferent projections to the hypothalamic paraventricular nucleus in the rat. Brain Res 379:200–203
Tanaka J, Hayashi Y, Shimamune S, Nomura M (1997) Ascending pathways from the nucleus of the solitary tract to the subfornical organ in the rat. Brain Res 777:237–241
Tanaka J, Hayashi Y, Watai T, Hori K, Nomura M (1998) Noradrenaline release in the rat subfornical organ area to blood pressure changes. Exp Neurol 152:303–306
Tanaka J, Hayashi Y, Nomura S, Miyakubo H, Okumura T, Sakamaki K (2001a) Angiotensinergic and noradrenergic mechanisms in the hypothalamic paraventricular nucleus participate in the drinking response induced by activation of the subfornical organ in rats. Behav Brain Res 118:117–122
Tanaka J, Hori K, Nomura M (2001b) Dipsogenic response induced by angiotensinergic pathways from the lateral hypothalamic area to the subfornical organ in rats. Behav Brain Res 118:111–116
Tanaka J, Miyakubo H, Nomura S, Sakamaki K, Okumura T, Hayashi Y (2001c) GABAergic modulation of neurons in the nucleus of the solitary tract with ascending projections to the subfornical organ in the rat. Brain Res 888:184–188
Tanaka J, Mashiko N, Kawakami A, Hatakenaka S, Fujisawa S, Nomura M (2002) The action of the A1 noradrenergic region on the activity of subfornical organ neurons in the rat. Neurosci Lett 332:41–44
Tanaka J, Kariya K, Nomura M (2003) Drinking attenuates the noradrenaline release in the lateral hypothalamic area induced by angiotensin II activation of the subfornical organ in rats. Behav Brain Res 140:49–55
Unger T, Bles F, Ganten D, Lang RE, Rettig R, Schwab NA (1983) GABAergic stimulation inhibits central actions of angiotensin II: pressor responses, drinking and release of vasopressin. Eur J Pharmacol 90:1–9
Ushigome A, Tanaka J, Kariya K, Nomura M (2002) Paraventricular noradrenergic systems participate in angiotensin II-induced drinking. Peptides 23:2169–2175
Zardetto-Smith AM, Watson CA (1987) A direct neural projection from the nucleus of the solitary tract to the subfornical organ in the rat. Neurosci Lett 80:163–166
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, M., Tanaka, J. Noradrenaline receptor mechanisms modulate the angiotensin II-induced water intake in the subfornical organ in rats. Exp Brain Res 235, 833–839 (2017). https://doi.org/10.1007/s00221-016-4844-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4844-9