Abstract
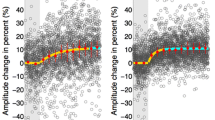
When we explore the visual environment around us, we produce sequences of very precise eye movements aligning the objects of interest with the most sensitive part of the retina for detailed visual processing. A copy of the impending motor command, the corollary discharge, is sent as soon as the first saccade in a sequence is ready to monitor the next fixation location and correctly plan the subsequent eye movement. Neurophysiological investigations have shown that chemical interference with the corollary discharge generates a distinct pattern of spatial errors on sequential eye movements, with similar results also from clinical and TMS studies. Here, we used saccadic inhibition to interfere with the temporal domain of the first of two subsequent saccades during a standard double-step paradigm. In two experiments, we report that the temporal interference on the primary saccade led to a specific error in the final landing position of the second saccade that was consistent with previous lesion and neurophysiological studies, but without affecting the spatial characteristics of the first eye movement. On the other hand, single-step saccades were differently influence by the flash, with a general undershoot, more pronounced for larger saccadic amplitude. These findings show that a flashed visual transient can disrupt saccadic updating in a double-step task, possibly due to the mismatch between the planned and the executed saccadic eye movement.






Similar content being viewed by others
References
Atsma J, Maij F, Corneil BD, Medendorp P (2014) No evidence for peri-saccadic mislocalization on suddenly cancelled saccades. J Neurosci 34(16):5497–5504
Bahcall D, Kowler E (1999) Illusory shifts in visual direction accompany adaptation of saccadic eye movements. Nature 400:864–866
Becker W, Jürgens R (1979) An analysis of the saccadic system by means of double-step stimuli. Vis Res 19:967–983
Benevento LA, Fallon JH (1975) The ascending projections of the superior colliculus in the rhesus monkey (Macaca mulatta). J Comp Neurol 160(3):339–361
Bompas A, Sumner P (2011) Saccadic inhibition reveals the timing of automatic and voluntary signals in the human brain. J Neurosci 31:12501–12512
Buonocore A, McIntosh RD (2008) Saccadic inhibition underlies the remote distractor effect. Exp Brain Res 191:117–122
Buonocore A, McIntosh RD (2012) Modulation of saccadic inhibition by distractor size and location. Vis Res 69:32–41
Buonocore A, McIntosh RD (2013) Attention modulates saccadic inhibition magnitude. Q J Exp Psychol (Hove) 66(6):1051–1059
Collins T, Rolfs M, Deubel H, Cavanagh P (2009) Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. J Vis 9(5):29.1–29.9
Donaldson IM (2000) The functions of the proprioceptors of the eye muscles. Philos Trans R Soc Lond B Biol Sci 355(1404):1685–1754
Duhamel JR, Goldberg ME, Fitzgibbon EJ, Sirigu A, Grafman J (1992) Saccadic dysmetria in a patient with a right frontoparietal lesion. The importance of corollary discharge for accurate spatial behaviour. Brain 115:1387–1402
Edelman JA, Xu KZ (2009) Inhibition of voluntary saccadic eye movement commands by abrupt visual onsets. J Neurophysiol 101:1222–1234
Findlay JM, Walker R (1999) A model of saccade generation based on parallel processing and competitive inhibition. Behav Brain Sci 22:661–675
Goldman-Rakic PS, Porrino LJ (1985) The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol 242:535–560
Grantyn A (1989) How visual inputs to the ponto-bulbar reticular formation are used in the synthesis of premotor signals during orienting. Prog Brain Res 80:159–170
Guillaume A (2012) Saccadic inhibition is accompanied by large and complex amplitude modulations when induced by visual backward masking. J Vis 12(6):5
Guthrie BL, Porter JD, Sparks DL (1983) Corollary discharge provides accurate eye position information to the oculomotor system. Science 221:1193–1195
Hamker FH, Zirnsak M, Ziesche A, Lappe M (2011) Computational models of spatial updating in peri-saccadic perception. Phil Trans R Soc B 366:554–571
Heide W, Blankenburg M, Zimmermann E, Kömpf D (1995) Cortical control of double-step saccades: implications for spatial orientation. Ann Neurol 38:739–748
Joiner W, Fitzgibbon E, Wurtz R (2010) Amplitudes and directions of individual saccades can be adjusted by corollary discharge. J Vis 10(2):22.1–22.12
Katschmarsky S, Cairney S, Maruff P, Wilson PH, Currie J (2001) The ability to execute saccades on the basis of efference copy: impairments in double-step saccade performance in children with developmental co-ordination disorder. Exp Brain Res 136(1):73–78
Lewis RF, Zee DS, Hayman MR, Tamargo RJ (2001) Oculomotor function in the rhesus monkey after deafferentation of the extraocular muscles. Exp Brain Res 141(3):349–358
Lynch J, Hoover J, Strick P (1994) Input to the primate frontal eye field from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Exp Brain Res 100:181–186
McIntosh RD, Buonocore A (2014) Saccadic inhibition can cause the remote distractor effect, but the remote distractor effect may not be a useful concept. J Vis 14(5):15
Medendorp WP (2011) Spatial constancy mechanisms in motor control. Phil Trans R Soc Lond B Biol Sci 366:476–491
Melcher D (2007) Predictive remapping of visual features precedes saccadic eye movements. Nat Neurosci 10:903–907
Melcher D (2011) Visual stability. Philos Trans R Soc Lond B Biol Sci 366:468–475
Melcher D, Colby CL (2008) Trans-saccadic perception. Trends Cogn Sci 12:466–473
Melcher D, Morrone MC (2003) Spatiotopic integration of visual motion across saccadic eye movements. Nat Neurosci 6:877–881
Morris AP, Chambers CD, Mattingley JB (2007) Parietal stimulation destabilizes spatial updating across saccadic eye movements. Proc Natl Acad Sci USA 104(21):9069–9074
Munoz DP, Wurtz RH (1993a) Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70:559–575
Munoz DP, Wurtz RH (1993b) Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol 70:576–589
Olivier E, Dorris MC, Munoz DP (1999) Lateral interactions in the superior colliculus, not an extended fixation zone, can account for the remote distractor effects (Commentary on Findlay & Walker). Behav Brain Sci 22:694–695
Pisella L, Mattingley JB (2004) The contribution of spatial remapping impairments to unilateral visual neglect. Neurosci Biobehav Rev 28:181–200
Poletti M, Burr DC, Rucci M (2013) Optimal multimodal integration in spatial localization. J Neurosci 33:14259–14268
Proske U, Gandevia SC (2012) The proprioceptive senses: their roles in signalling body shape, body position and movement, and muscle force. Physiol Rev 92:1651–1697
Reingold EM, Stampe DM (2002) Saccadic inhibition in voluntary and reflexive saccades. J Cogn Neurosci 14:371–388
Rizzolatti G, Buchtel HA, Camarda R, Scandolara C (1980) Neurons with complex visual properties in the superior colliculus of the macaque monkey. Exp Brain Res 38(1):37–42
Sommer MA, Wurtz RH (1998) Frontal eye field neurons orthodromically activated from the superior colliculus. J Neurophysiol 80:3331–3335
Sommer MA, Wurtz RH (2002) A pathway in primate brain for internal monitoring of movements. Science 296:1480–1482
Sommer MA, Wurtz RH (2004) What the brainstem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol 91:1403–1423
Sparks DL, Hartwich-Young R (1989) The deeper layers of the superior colliculus. In: Wurtz RH, Goldberg ME (eds) Rev Oculomotor Res. The neurobiology of saccadic eye movements. Elsevier Science Publishers, Amsterdam, pp 213–255
Sperry RW (1950) Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43:482–489
Walker R, McSorley E (2006) The parallel programming of voluntary and reflexive saccades. Vis Res 46(13):2082–2093
Wurtz RH (2008) Neuronal mechanisms of visual stability. Vis Res 48:2070–2089
Wurtz RH, Joiner WM, Berman RA (2011) Neuronal mechanisms for visual stability: progress and problems. Philos Trans R Soc Lond B Biol Sci 366:492–503
Zimmermann E, Fink G, Cavanagh P (2013a) Perifoveal spatial compression. J Vis 13(21):1–9
Zimmermann E, Morrone MC, Fink GR, Burr D (2013b) Spatiotopic neural representations develop slowly across saccades. Curr Biol 5:R193–R194
Zimmerman E, Born S, Fink GR, Cavanagh P (2014) Masking produces compression of space and time in the absence of eye movements. J Neurophysiol. doi:10.1152/jn.00156.2014
Acknowledgments
This research was funded by a European Research Council Starting Grant award (Grant Agreement No. 313658) to D.M. and a research fellowship awarded to A.B. by the Comune di Rovereto.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards and the guidelines of the University of Trento Ethics Committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buonocore, A., Melcher, D. Disrupting saccadic updating: visual interference prior to the first saccade elicits spatial errors in the secondary saccade in a double-step task. Exp Brain Res 233, 1893–1905 (2015). https://doi.org/10.1007/s00221-015-4261-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4261-5