Abstract
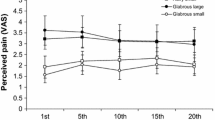
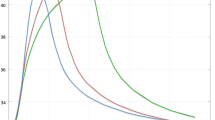
Ramp-and-hold heat stimulation with a Peltier thermode is a standard procedure for quantitative sensory testing of human pain sensitivity. Because myelinated and unmyelinated nociceptive afferents respond preferentially to changing and steady temperatures, respectively, ramp-and-hold heat stimulation could assess processing of input from A-delta nociceptors early and C nociceptors late during prolonged thermal stimulation. In order to evaluate the progression from dynamic change to a steady temperature during prolonged Peltier stimulation, recordings of temperatures at the probe–skin interface were obtained. First, recordings of temperature during contact-and-hold stimulation (solenoid powered delivery of a preheated thermode to the skin) provided an evaluation of heat dissipation from the beginning of stimulation, uncontaminated by ramping. The heat-sink effect lasted up to 8 s and accounted in part for a slow increase in pain intensity for stimulus durations of 1–16 s and stimulus intensities of 43–59 °C. Recordings during longer periods of stimulation showed that feedback-controlled Peltier stimulation generated oscillations in temperature that were tracked for up to 75 s by subjects’ continuous ratings of pain. During 120-s trials, sensitization of pain was observed over 45 s after the oscillations subsided. Thus, long-duration stimulation can be utilized to evaluate sensitization, presumably of C nociception, when not disrupted by oscillations in thermode temperature (e.g., those inherent to feedback control of Peltier stimulation). In contrast, sensitization was not observed during 130.5 s of stimulation with alternately increasing and decreasing temperatures that repeatedly activated A-delta nociceptors.






Similar content being viewed by others
References
Adriaensen H, Gybels J, Handwerker HO, Van HJ (1984) Nociceptor discharges and sensations due to prolonged noxious mechanical stimulation–a paradox. Hum Neurobiol 3:53–58
Hanai F (2000) Effect of electrical stimulation of peripheral nerves on neuropathic pain. Spine (Phila Pa 1976) 25:1886–1892
Hashmi JA, Davis KD (2010) Effects of temperature on heat pain adaptation and habituation in men and women. Pain 151:737–743
Johnson MI, Tabasam G (2003) An investigation into the analgesic effects of interferential currents and transcutaneous electrical nerve stimulation on experimentally induced ischemic pain in otherwise pain-free volunteers. Phys Ther 83:208–223
Kenshalo DR Jr, Anton F, Dubner R (1989) The detection and perceived intensity of noxious thermal stimuli in monkey and in human. J Neurophysiol 62:429–436
Koyama Y, Koyama T, Kroncke AP, Coghill RC (2004) Effects of stimulus duration on heat induced pain: the relationship between real-time and post-stimulus pain ratings. Pain 107:256–266
Lee KH, Chung JM, Willis WD Jr (1985) Inhibition of primate spinothalamic tract cells by TENS. J Neurosurg 62:276–287
Liu XG, Morton CR, Azkue JJ, Zimmermann M, Sandkuhler J (1998) Long-term depression of C-fibre-evoked spinal field potentials by stimulation of primary afferent A delta-fibres in the adult rat. Eur J Neurosci 10:3069–3075
Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150:971–979
Nielsen J, Arendt-Nielsen L (1998a) The importance of stimulus configuration for temporal summation of first and second pain to repeated heat stimuli. Eur J Pain 2:329–341
Nielsen J, Arendt-Nielsen L (1998b) The influence of rate of temperature change and peak stimulus duration on pain intensity and quality. Somatosens Mot Res 15:220–229
Pantaleao MA, Laurino MF, Gallego NL, Cabral CM, Rakel B, Vance C, Sluka KA, Walsh DM, Liebano RE (2011) Adjusting pulse amplitude during transcutaneous electrical nerve stimulation (TENS) application produces greater hypoalgesia. J Pain 12:581–590
Pertovaara A (1999) The influence of stimulus temperature rise rate, adapting temperature, and stimulus duration on suprathreshold responses evoked by noxious heat in the glabrous skin of the limb. Comparison of neuronal discharge in the rat spinal dorsal horn with human sensations. Exp Brain Res 126:482–494
Pertovaara A, Kojo I (1985) Influence of the rate of temperature change on thermal thresholds in man. Exp Neurol 87:439–445
Pertovaara A, Kauppila T, Hamalainen MM (1996) Influence of skin temperature on heat pain threshold in humans. Exp Brain Res 107:497–503
Price D (1988) Psychological and neural mechanisms of pain. Raven Press, New York
Price DD, Hu JW, Dubner R, Gracely RH (1977) Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain 3:57–68
Sandkuhler J, Gruber-Schoffnegger D (2012) Hyperalgesia by synaptic long-term potentiation (LTP): an update. Curr Opin Pharmacol 12:18–27
Sjolund BH (1985) Peripheral nerve stimulation suppression of C-fiber-evoked flexion reflex in rats. Part 1: parameters of continuous stimulation. J Neurosurg 63:612–616
Vierck CJ, Cannon RL, Fry G, Maixner WL, Whitsel B (1997) Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol 78:992–1002
Vierck CJ, Riley JL III, Wong F, King CD, Mauderli AP (2010) Psychophysical demonstration of bidirectional pain modulation (sensitization and desensitization) by ascending or descending progressions of thermal stimulus intensity. Brain Res 1347:58–64
Wahren LK, Torebjork E, Jorum E (1989) Central suppression of cold-induced C fibre pain by myelinated fibre input. Pain 38:313–319
Wong F, Vierck CJ, Riley JL III, King C, Mauderli AP (2010) A new thermal stimulation method for human psychophysical studies: pain intensity clamping. J Neurosci Methods 188:83–88
Yarnitsky D, Ochoa JL (1990) Release of cold-induced burning pain by block of cold-specific afferent input. Brain 113(Pt 4):893–902
Yeomans DC, Proudfit HK (1996) Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain 68:141–150
Yucel A, Andersen OK, Nielsen J, rendt-Nielsen L (2002) Heat hyperalgesia in humans: assessed by different stimulus temperature profiles. Eur J Pain 6:357–364
Zachariou V, Goldstein BD, Yeomans DC (1997) Low but not high rate noxious radiant skin heating evokes a capsaicin-sensitive increase in spinal cord dorsal horn release of substance P. Brain Res 752:143–150
Acknowledgments
Alison Riley is acknowledged for recruiting and testing subjects. This research is supported by grant #AG039659 from the National Institute of Aging.
Conflict of interest
The authors declare that they have no conflict of interest with the funding agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vierck, C.J., Mauderli, A.P. & Riley III, J.L. Relationships between the intensity and duration of Peltier heat stimulation and pain magnitude. Exp Brain Res 225, 339–348 (2013). https://doi.org/10.1007/s00221-012-3375-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3375-2