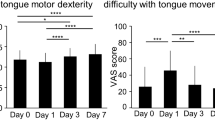
Abstract
Corticomotor pathways may undergo neuroplastic changes in response to acquisition of new motor skills. Little is known about the motor control strategies for learning new tongue tasks. The aim of this study was to investigate the longitudinal effect of novel tongue-task training on corticomotor neuroplasticity. Thirteen healthy, right-handed men, aged 24–35 years (mean age ± SD: 27.3 ± 0.3 years), performed a training task consisting of standardized tongue protrusion onto a force transducer. The tongue task consisted of a relax–protrude–hold–relax cycle with 1.0 N as the target at the hold phase lasting for 1.5 s. Subjects repeated this task for 1 h. Functional magnetic resonance imaging was carried out before the tongue-task training (baseline), 1-h after the training, and one-day and one-week follow-up. During scanning, the subjects performed tongue protrusion in blocks interspersed with rest. A region-of-interest (ROI) approach and an explorative search were implemented for the analysis of corticomotor activity across conditions. All subjects completed the tongue-task training (mean success rate 43.0 ± 13.2%). In the baseline condition, tongue protrusion resulted in bilateral activity in regions most typically associated with a motor task including medial frontal gyrus (supplementary motor area [SMA]), precentral gyrus (tongue motor cortex), putamen, thalamus, and cerebellum. The ROI analysis revealed increased activity in the precentral gyrus already 1 h post-training. One day after the training, increased activity was observed in the precentral gyrus, SMA, putamen, and cerebellum. No increase was found 1 week after training. Correlation analyses between changes in success rates and changes in the numbers of voxels showed robust associations for left Area 4a in primary motor cortex 1 h, 1 day, and 1 week after the tongue-task training and for the left Area 4p in primary motor cortex and the left lateral premotor cortex 1 day after the training. In the unrestricted analysis, increased activity was found in the parahippocampal gyrus 1 h after the tongue-task training and remained for a week. Decreased activity was found in right post-central and middle frontal gyri 1 h and 1 week post-training. The results verified the involvement of specific corticomotor areas in response to tongue protrusion. Short-term tongue-task training was associated with longer-lasting (up to 1 week) changes in motor-related brain activity. The results suggested that primary motor areas are involved in the early and late stages, while other motor areas mainly are engaged in the later stage of corticomotor neuroplasticity of the tongue.



Similar content being viewed by others
References
Ashburner J, Andersson JL, Friston KJ (1999) High-dimensional image registration using symmetric priors. Neuroimage 9:619–628
Baad-Hansen L, Blicher JU, Lapitskaya N, Nielsen JF, Svensson P (2009) Intra-cortical excitability in healthy human subjects after tongue training. J Oral Rehabil 36:427–434
Beisteiner R, Erdler M, Mayer D, Gartus A, Edward V, Kaindl T, Golaszewski S, Lindinger G, Deecke L (1999) A marker for differentiation of capabilities for processing of musical harmonies as detected by magnetoencephalography in musicians. Neurosci Lett 277:37–40
Boudreau S, Romaniello A, Wang K, Svensson P, Sessle BJ, Arendt-Nielsen L (2007) The effects of intra-oral pain on motor cortex neuroplasticity associated with short-term novel tongue-protrusion training in humans. Pain 132:169–178
Boudreau SA, Hennings K, Svensson P, Sessle BJ, Arendt-Nielsen L (2010) The effects of training time, sensory loss and pain on human motor learning. J Oral Rehabil 37(9):704–718
Brett M, Anton J, Valabregue R, Poline J (2002) Region of interest analysis using an SPM toolbox [Abstract: 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan]. Neuroimage 16
Buchner H, Kauert C, Radermacher I (1995) Short-term changes of finger representation at the somatosensory cortex in humans. Neurosci Lett 198:57–59
Cheyne D, Kristeva R, Deecke L (1991) Homuncular organization of human motor cortex as indicated by neuromagnetic recordings. Neurosci Lett 122:17–20
Classen J, Liepert J, Wise SP, Hallett M, Cohen LG (1998) Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79:1117–1123
Corfield DR, Murphy K, Josephs O, Fink GR, Frackowiak RS, Guz A, Adams L, Turner R (1999) Cortical and subcortical control of tongue movement in humans: a functional neuroimaging study using fMRI. J Appl Physiol 86:1468–1477
Dellow PG, Lund JP (1971) Evidence for central timing of rhythmical mastication. J Physiol 215:1–13
Dinardo LA, Travers JB (1994) Hypoglossal neural activity during ingestion and rejection in the awake rat. J Neurophysiol 72:1181–1191
Doyon J, Benali H (2005) Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15:161–167
Dubner R, Sessle BJ, Storey AT (1978) The neural basis of oral and facial function. Plenum, New York
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335
Ernberg M, Serra E, Baad-Hansen L, Svensson P (2009) Influence of topical anaesthesia on the corticomotor response to tongue training. Arch Oral Biol 54:696–704
Floyer-Lea A, Matthews PM (2005) Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol 94:512–518
Foerster O (1936) Motorische felder und bahnen. Springer, Berlin
Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline J (2000) To smooth or not to smooth? Bias and efficiency in fMRI time-series analysis. Neuroimage 12:196–208
Gavazzi C, Nave RD, Petralli R, Rocca MA, Guerrini L, Tessa C, Diciotti S, Filippi M, Piacentini S, Mascalchi M (2007) Combining functional and structural brain magnetic resonance imaging in Huntington disease. J Comput Assist Tomogr 31:574–580
Giorgio A, Portaccio E, Stromillo ML, Marino S, Zipoli V, Battaglini M, Blandino A, Bartolozzi ML, Siracusa G, Amato MP, De Stefano N (2010) Cortical functional reorganisation and its relationship with brain structural damage in patients with benign multiple sclerosis. Mult Scler [Epub ahead of print]
Grafton ST, Woods RP, Mazziotta JC, Phelps ME (1991) Somatotopic mapping of the primary motor cortex in humans: activation studies with cerebral blood flow and positron emission tomography. J Neurophysiol 66:735–743
Grafton ST, Woods RP, Mazziotta JC (1993) Within-arm somatotopy in human motor areas determined by positron emission tomography imaging of cerebral blood flow. Exp Brain Res 95:172–176
Halkjaer L, Melsen B, McMillan AS, Svensson P (2006) Influence of sensory deprivation and perturbation of trigeminal afferent fibers on corticomotor control of human tongue musculature. Exp Brain Res 170:199–205
Hari R, Karhu J, Hamalainen M, Knuutila J, Salonen O, Sams M, Vilkman V (1993) Functional organization of the human first and second somatosensory cortices: a neuromagnetic study. Eur J Neurosci 5:724–734
Hesselmann V, Sorger B, Lasek K, Guntinas-Lichius O, Krug B, Sturm V, Goebel R, Lackner K (2004) Discriminating the cortical representation sites of tongue and up movement by functional MRI. Brain Topogr 16:159–167
Hiiemae KM, Palmer JB (2003) Tongue movements in feeding and speech. Crit Rev Oral Biol Med 14:413–429
Ioffe ME (2004) Brain mechanisms for the formation of new movements during learning: the evolution of classical concepts. Neurosci Behav Physiol 34:5–18
Jean A (1984a) Brainstem organization of the swallowing network. Brain Behav Evol 25:109–116
Jean A (1984b) Control of the central swallowing program by inputs from the peripheral receptors. A review. J Auton Nerv Syst 10:225–233
Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG (1998) The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA 95:861–868
Kawashima R, Itoh H, Ono S, Satoh K, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Yanagisawa T et al (1995) Activity in the human primary motor cortex related to arm and finger movements. Neuroreport 6:238–240
Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M (2004) Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci 24:628–633
Koeneke S, Lutz K, Herwig U, Ziemann U, Jancke L (2006) Extensive training of elementary finger tapping movements changes the pattern of motor cortex excitability. Exp Brain Res 174:199–209
Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG (2003) Motor learning elicited by voluntary drive. Brain 126:866–872
Loubinoux I, Carel C, Alary F, Boulanouar K, Viallard G, Manelfe C, Rascol O, Celsis P, Chollet F (2001) Within-session and between-session reproducibility of cerebral sensorimotor activation: a test–retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab 21:592–607
Lowe AA (1980) The neural regulation of tongue movements. Prog Neurobiol 15:295–344
Lund JP (1991) Mastication and its control by the brain stem. Crit Rev Oral Biol Med 2:33–64
Maldjian JA, Gottschalk A, Patel RS, Detre JA, Alsop DC (1999) The sensory somatotopic map of the human hand demonstrated at 4 Tesla. Neuroimage 10:55–62
Martin RE, Murray GM, Kemppainen P, Masuda Y, Sessle BJ (1997) Functional properties of neurons in the primate tongue primary motor cortex during swallowing. J Neurophysiol 78:1516–1530
Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM, Sessle BJ (1999) Features of cortically evoked swallowing in the awake primate (Macaca fascicularis). J Neurophysiol 82:1529–1541
Miller AJ (2002) Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med 13:409–425
Muellbacher W, Boroojerdi B, Ziemann U, Hallett M (2001) Analogous corticocortical inhibition and facilitation in ipsilateral and contralateral human motor cortex representations of the tongue. J Clin Neurophysiol 18:550–558
Murray GM, Sessle BJ (1992a) Functional properties of single neurons in the face primary motor cortex of the primate. I. Input and output features of tongue motor cortex. J Neurophysiol 67:747–758
Murray GM, Sessle BJ (1992b) Functional properties of single neurons in the face primary motor cortex of the primate. II. Relations with trained orofacial motor behavior. J Neurophysiol 67:759–774
Murray GM, Sessle BJ (1992c) Functional properties of single neurons in the face primary motor cortex of the primate. III. Relations with different directions of trained tongue protrusion. J Neurophysiol 67:775–785
Murray GM, Lin LD, Moustafa EM, Sessle BJ (1991) Effects of reversible inactivation by cooling of the primate face motor cortex on the performance of a trained tongue-protrusion task and a trained biting task. J Neurophysiol 65:511–530
Nakamura Y, Katakura N (1995) Generation of masticatory rhythm in the brainstem. Neurosci Res 23:1–19
Nakamura A, Yamada T, Goto A, Kato T, Ito K, Abe Y, Kachi T, Kakigi R (1998) Somatosensory homunculus as drawn by MEG. Neuroimage 7:377–386
Nakasato N, Itoh H, Hatanaka K, Nakahara H, Kanno A, Yoshimoto T (2001) Movement-related magnetic fields to tongue protrusion. Neuroimage 14:924–935
Pascual-Leone A, Grafman J, Hallett M (1994) Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science 263:1287–1289
Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M (1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74:1037–1045
Penfield W, Boldrey E (1937) Somatic motor and sensory representation in the cerebral cortex as studied by electrical stimulation. Brain 60:389–443
Rao SM, Binder JR, Hammeke TA, Bandettini PA, Bobholz JA, Frost JA, Myklebust BM, Jacobson RD, Hyde JS (1995) Somatotopic mapping of the human primary motor cortex with functional magnetic resonance imaging. Neurology 45:919–924
Rosenkranz K, Nitsche MA, Tergau F, Paulus W (2000) Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulation in the human. Neurosci Lett 296:61–63
Sakai K, Watanabe E, Onodera Y, Itagaki H, Yamamoto E, Koizumi H, Miyashita Y (1995) Functional mapping of the human somatosensory cortex with echo-planar MRI. Magn Reson Med 33:736–743
Sanes JN, Donoghue JP (2000) Plasticity and primary motor cortex. Annu Rev Neurosci 23:393–415
Sawczuk A, Mosier KM (2001) Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med 12:18–37
Schenck CH, Mahowald MW (1991) Injurious sleep behavior disorders (parasomnias) affecting patients on intensive care units. Intensive Care Med 17:219–224
Sessle BJ, Yao D, Nishiura H, Yoshino K, Lee JC, Martin RE, Murray GM (2005) Properties and plasticity of the primate somatosensory and motor cortex related to orofacial sensorimotor function. Clin Exp Pharmacol Physiol 32:109–114
Sessle BJ, Adachi K, Avivi-Arber L, Lee J, Nishiura H, Yao D, Yoshino K (2007) Neuroplasticity of face primary motor cortex control of orofacial movements. Arch Oral Biol 52:334–337
Shinagawa H, Ono T, Ishiwata Y, Honda E, Sasaki T, Taira M, Iriki A, Kuroda T (2003) Hemispheric dominance of tongue control depends on the chewing-side preference. J Dent Res 82:278–283
Smith A (1992) The control of orofacial movements in speech. Crit Rev Oral Biol Med 3:233–267
Svensson P, Romaniello A, Arendt-Nielsen L, Sessle BJ (2003) Plasticity in corticomotor control of the human tongue musculature induced by tongue-task training. Exp Brain Res 152:42–51
Svensson P, Romaniello A, Wang K, Arendt-Nielsen L, Sessle BJ (2006) One hour of tongue-task training is associated with plasticity in corticomotor control of the human tongue musculature. Exp Brain Res 173:165–173
Tessa C, Lucetti C, Diciotti S, Baldacci F, Paoli L, Cecchi P, Giannelli M, Ginestroni A, Del Dotto P, Ceravolo R, Vignali C, Bonuccelli U, Mascalchi M (2010) Decreased and increased cortical activation coexist in de novo Parkinson’s disease. Exp Neurol 224:299–306
Travers JB, DiNardo LA, Karimnamazi H (2000) Medullary reticular formation activity during ingestion and rejection in the awake rat. Exp Brain Res 130:78–92
Ungerleider LG, Doyon J, Karni A (2002) Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem 78:553–564
Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ (2010) Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 68:359–367
Warraich Z, Kleim JA (2010) Neural plasticity: the biological substrate for neurorehabilitation. PMR 2:S208–S219
Wassermann EM, McShane LM, Hallett M, Cohen LG (1992) Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol 85:1–8
Watanabe J, Sugiura M, Miura N, Watanabe Y, Maeda Y, Matsue Y, Kawashima R (2004) The human parietal cortex is involved in spatial processing of tongue movement-an fMRI study. Neuroimage 21:1289–1299
Westberg K, Clavelou P, Sandstrom G, Lund JP (1998) Evidence that trigeminal brainstem interneurons form subpopulations to produce different forms of mastication in the rabbit. J Neurosci 18:6466–6479
Wiesenfeld Z, Halpern BP, Tapper DN (1977) Licking behavior: evidence of hypoglossal oscillator. Science 196:1122–1124
Wilson SA, Thickbroom GW, Mastaglia FL (1993) Transcranial magnetic stimulation mapping of the motor cortex in normal subjects. The representation of two intrinsic hand muscles. J Neurol Sci 118:134–144
Yamamura K, Narita N, Yao D, Martin RE, Masuda Y, Sessle BJ (2002) Effects of reversible bilateral inactivation of face primary motor cortex on mastication and swallowing. Brain Res 944:40–55
Yao D, Yamamura K, Narita N, Martin RE, Murray GM, Sessle BJ (2002a) Neuronal activity patterns in primate primary motor cortex related to trained or semiautomatic jaw and tongue movements. J Neurophysiol 87:2531–2541
Yao D, Yamamura K, Narita N, Murray GM, Sessle BJ (2002b) Effects of reversible cold block of face primary somatosensory cortex on orofacial movements and related face primary motor cortex neuronal activity. Somatosens Mot Res 19:261–271
Zhang Y, Boudreau S, Wang M, Wang K, Sessle B, Arendt-Nielsen L, Svensson P (2010) Effects of periodontal afferent inputs on corticomotor excitability in humans. J Oral Rehabil 37:39–47
Ziemann U, Muellbacher W, Hallett M, Cohen LG (2001) Modulation of practice-dependent plasticity in human motor cortex. Brain 124:1171–1181
Acknowledgments
BJS involvement was supported by CIHR grant MT-4918.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arima, T., Yanagi, Y., Niddam, D.M. et al. Corticomotor plasticity induced by tongue-task training in humans: a longitudinal fMRI study. Exp Brain Res 212, 199–212 (2011). https://doi.org/10.1007/s00221-011-2719-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2719-7