Abstract
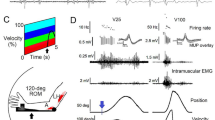
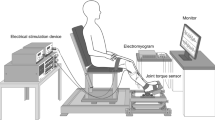
Simultaneous contraction of agonist and antagonist muscles acting about a joint influences joint stiffness and stability. Although several studies have shown that reflexes in the muscle lengthened by a joint perturbation are modulated during co-contraction, little attention has been given to reflex regulation in the antagonist (shortened) muscle. The goal of the present study was to determine whether co-contraction gives rise to altered reflex regulation across the joint by examining reflexes in the muscle shortened by a joint perturbation. Reflexes were recorded from electromyographic activity in elbow flexors and extensors while positional perturbations to the elbow joint were applied. Perturbations were delivered during isolated activation of the flexor or extensor muscles as well as during flexor and extensor co-contraction. Across the group, the shortening reflex in the elbow extensor switched from suppression during isolated extensor muscle activation to facilitation during co-contraction. The shortening reflex in the elbow flexor remained suppressive during co-contraction but was significantly smaller compared to the response obtained during isolated elbow flexor activation. This response in the shortened muscle was graded by the level of activation in the lengthened muscle. The lengthening reflex did not change during co-contraction. These results support the idea that reflexes are regulated across multiple muscles around a joint. We speculate that the facilitatory response in the shortened muscle arises through a fast-conducting oligosynaptic pathway involving Ib interneurons.





Similar content being viewed by others
References
Akazawa K, Milner TE, Stein RB (1983) Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol 49:16–27
Angel RW, Lewitt PA (1978) Unloading and shortening reactions in Parkinson’s disease. J Neurol Neurosurg Psychiatry 41:919–923
Bawa P, Sinkjaer T (1999) Reduced short and long latency reflexes during voluntary tracking movement of the human wrist joint. Acta Physiol Scand 167:241–246
Berardelli A, Hallett M (1984) The shortening reaction of human tibialis anterior. Neurology 34:242–246
Carter RR, Crago PE, Gorman PH (1993) Nonlinear stretch reflex interaction during cocontraction. J Neurophysiol 69:943–952
Colebatch JG, Gandevia SC, McCloskey DI, Potter EK (1979) Subject instruction and long-latency reflex responses to muscle stretch. J Physiol 292:527–534
Crago PE, Houk JC, Hasan Z (1976) Regulatory actions of human stretch reflex. J Neurophysiol 39:925–935
Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB (2003) Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126:495–507
Day SJ, Hulliger M (2001) Experimental simulation of cat electromyogram: evidence for algebraic summation of motor-unit action-potential trains. J Neurophysiol 86:2144–2158
Dietz V, Bischer M, Faist M, Trippel M (1990) Amplitude modulation of the human quadriceps tendon jerk reflex during gait. Exp Brain Res 82:211–213
Dietz V, Discher M, Trippel M (1994) Task-dependent modulation of short- and long-latency electromyographic responses in upper limb muscles. Electroencephalogr Clin Neurophysiol 93:49–56
Doemges F, Rack PM (1992) Task-dependent changes in the response of human wrist joints to mechanical disturbance. J Physiol 447:575–585
Enoka RM (1997) Neural strategies in the control of muscle force. Muscle Nerve S5:66–69
Enriquez-Denton M, Nielsen JB, Perreault MC, Morita H, Petersen N, Hultborn H (2000) Presynaptic control of transmission along the pathway mediating disynaptic reciprocal inhibition in the cat. J Physiol 526:623–637
Gottlieb GL, Myklebust BM, Penn RD, Agarwal GC (1982) Reciprocal excitation of muscle antagonists by the primary afferent pathway. Exp Brain Res 46:454–456
Hammond PH (1956) The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. Society 17P–18P
Hultborn H, Jankowska E, Lindstrom S (1971) Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurons. J Physiol 215:591–612
Hultborn H, Illert M, Santini M (1976) Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. II. Effects from segmental flexor reflex pathways. Acta Physiol Scand 96:351–367
Humphrey DR (1982) Separate cell systems in the motor cortex of the monkey for the control of joint movement and of joint stiffness. Electroencephalogr Clin Neurophysiol 36:393–408
Humphrey DR, Reed DJ (1983) Separate cortical systems for control of joint movement and joint stiffness: reciprocal activation and coactivation of antagonist muscles. Adv Neurol 39:347–372
Jankowska E, Padel Y, Tanaka R (1976) Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol 258:467–487
Jankowska E, Johannisson T, Lipski J (1981) Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol 310:381–402
Johannsen P, Christensen LOD, Sinkjaer T, Nielsen JB (2001) Cerebral functional anatomy of voluntary contractions of ankle muscles in man. J Physiol 535:397–406
Katz R, Penicaud A, Rossi A (1991) Reciprocal Ia inhibition between elbow flexors and extensors in the human. J Physiol 437:269–286
Kornecki S (1992) Mechanism of muscular stabilization process in joints. J Biomech 25:235245
Krutky MA, Ravichandran VJ, Trumbower RD, Perreault EJ (2010) Interactions between limb and environmental mechanics influence stretch reflex sensitivity in the human arm. J Neurophysiol 103:429–440
Lacquaniti F, Borghese NA, Carrozzo M (1991) Transient reversal of the stretch reflex in human arm muscles. J Neurophysiol 66:939–954
Lance JW, Degail P (1965) Spread of phasic muscle reflexes in normal and spastic subjects. J Neurol Neurosurg Psychiatry 28:328–334
Laporte Y, Lloyd DPC (1952) Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol 169:609–621
Lewis GN, MacKinnon CD, Perreault EJ (2006) The effect of task instruction on the excitability of spinal and supraspinal reflex pathways projecting to the biceps muscle. Exp Brain Res 174:413–425
Matthews PBC (1986) Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol 374:73–90
Milner TE, Cloutier C (1993) Compensation for mechanically unstable loading in voluntary wrist movement. Exp Brain Res 94:522–532
Milner TE, Cloutier C (1998) Damping of the wrist joint during voluntary movement. Exp Brain Res 122:309–317
Miscio G, Pisano F, Del Conte C, Pianca D, Colombo R, Schieppati M (2001) The shortening reaction of forearm muscles: the influence of central set. Clin Neurophysiol 112:884–894
Myklebust BM, Gottlieb GL, Penn RD, Agarwal GC (1982) Reciprocal excitation of antagonistic muscles as a differentiating feature of spasticity. Ann Neurol 12:367–374
Nicolas G, Marchand-Pauvert V, Burke D, Pierrot-Deseilligny E (2001) Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans. J Physiol 533:903–919
Nielsen J, Kagamihara Y (1993) The regulation of presynaptic inhibition during co-contraction of antagonist muscles in man. J Physiol 464:575–593
Nielsen JB, Pierrot-Deseilligny E (1996) Evidence of facilitation of soleus-coupled Renshaw cells during voluntary co-contraction of antagonistic ankle muscles in man. J Physiol 493:603–611
Nielsen J, Petersen N, Deuschl G, Ballegaard M (1993) Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol 471:223–243
Nielsen J, Sinkjaer T, Toft E, Kagamihara Y (1994) Segmental reflexes and ankle joint stiffness during co-contraction of antagonistic ankle muscles in man. Exp Brain Res 102:350–358
Osu R, Franklin DW, Kato H, Gomi H, Domen K, Yoshioka T, Kawato M (2002) Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. J Neurophysiol 88:991–1004
Perreault EJ, Chen K, Trumbower RD, Lewis GN (2008) Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination. J Neurophysiol 99:2101–2113
Pierrot-Deseilligny E (1996) Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneruons. Prog Neurobiol 48:489–517
Pierrot-Deseilligny E, Bergego C, Katz R (1982) Reversal in cutaneous control of Ib pathways during human voluntary contraction. Brain Res 233:400–403
Pruszynski JA, Kurtzer I, Scott SH (2008) Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol 100:224–238
Rothwell JC, Traub MM, Marsden CD (1980) Influence of voluntary intent on the human long-latency stretch reflex. Nature 286:496–498
Selen LPJ, Beek PJ, Van Dieen JH (2006) Impedance is modulated to meet accuracy demands during goal-directed arm movements. Exp Brain Res 172:129–138
Shemmell J, Je HA, Perreault EJ (2009) The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci 29:13255–13263
Smith AM (1981) The coactivation of antagonist muscles. Can J Physiol Pharmacol 59:733–747
Solomonow M, Baratta R, Bernardi M, Zhou B, Lu Y, Zhu M, Acierno S (1994) Surface and wire EMG crosstalk in neighbouring muscles. J Electromyogr Kinesiol 4:131–142
Xia R, Rymer WZ (2005) Reflex reciprocal facilitation of antagonist muscles in spinal cord injury. Spinal Cord 43:14–21
Acknowledgments
The authors would like to thank Tiffany Ceja for her technical assistance and Krista Settle for her contributions to data collection. The project was supported by NIH grant R01 NS053813 to EJP and a fellowship from the Brinson Foundation awarded to GNL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lewis, G.N., MacKinnon, C.D., Trumbower, R. et al. Co-contraction modifies the stretch reflex elicited in muscles shortened by a joint perturbation. Exp Brain Res 207, 39–48 (2010). https://doi.org/10.1007/s00221-010-2426-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2426-9