Abstract
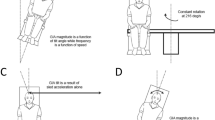
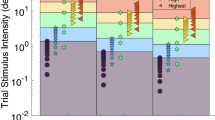
We had recumbent subjects (n = 7) indicate the amplitude of imposed, passive yaw–axis body rotations in the 0, 1, and 1.8 g background force levels generated during parabolic flight maneuvers. The blindfolded subject, restrained in a cradle, aligned a gravity-neutral pointer with the subjective vertical while in an initial position and then tried to keep it aligned with the same external direction during a body rotation, lasting less than 1.5 s about the z-axis 30°, 60°, or 120° in amplitude. All the rotations were above semicircular threshold levels for eliciting perception of angular displacement under terrestrial test conditions. In 1 and 1.8 g test conditions, subjects were able to indicate both the subjective vertical and the amplitude of the body rotation reasonably accurately. By contrast in 0 g, when indicating the subjective vertical, they aligned the pointer with the body midline and kept it nearly aligned with their midline during the subsequent body tilts. They also reported feeling supine throughout the 0 g test periods. The attenuation of apparent self-displacement in 0 g is discussed in terms of (1) a possible failure of integration of semicircular canal velocity signals, (2) a contribution of somatosensory pressure and contact cues, and (3) gravicentric versus body-centric reference frames. The significance of the findings for predicting and preventing motion sickness and disorientation in orbital space flight and in rotating artificial gravity environments is discussed.


Similar content being viewed by others
References
Angelaki DE, Hess BJ (1995) Inertial representation of angular motion in the vestibular system of the rhesus monkeys. II. Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol 73:1729–1751
Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJ (1999) Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci 19:316–327
Beritov IS (1957) Spatial projection of objects perceived in the external environment by means of labyrinthine receptors. Fiziol Zh SSSR Im I M Sechenova 43:600–610
Beritov IS (1959) Mechanism of spatial orientation in man. Zh Vyssh Nerv Deiat Im I P Pavlova 9:3–13
Bloomberg J, Jones GM, Segal B, McFarlane S, Soul J (1988) Vestibular-contingent voluntary saccades based on cognitive estimates of remembered vestibular information. Adv Otorhinolaryngol 41:71–75
Bloomberg J, Jones GM, Segal B (1991a) Adaptive modification of vestibularly perceived rotation. Exp Brain Res 84:47–56
Bloomberg J, Jones GM, Segal B (1991b) Adaptive plasticity in the gaze stabilization synergy of slow and saccadic eye movement. Exp Brain Res 84:35–46
Bortolami S, Pierobon A, DiZio P, Lackner J (2006a) Localization of the subjective vertical during roll, pitch, and recumbent yaw body tilt. Exp Brain Res 173:364–373
Bortolami SB, Rocca S, DiZio P, Lackner J (2006b) Mechanisms of human static spatial orientation. Exp Brain Res 173:374–388
Brown JE, Yates BJ, Taube JS (2002) Does the vestibular system contribute to head direction cell activity in the rat? Physiol Behav 77:743–748
Brown JE, Card JP, Yates BJ (2005) Polysynaptic pathways from the vestibular nuclei to the lateral mammillary nucleus of the rat: substrates for vestibular input to head direction cells. Exp Brain Res 161:47–61
Bryan AS, Bortolami SB, Ventura J, Dizio P, Lackner JR (2007) Influence of gravitoinertial force level on the subjective vertical during recumbent yaw axis body tilt. Exp Brain Res 183:389–397
Calton JL, Taube JS (2005) Degradation of head direction cell activity during inverted locomotion. J Neurosci 25:2420–2428
Clark B, Graybiel A (1949) The effect of angular acceleration on sound localization (The audiogyral illusion). J Psychol 28:235–244
Correia MJ, Hixson WC, Niven JI (1968) On predictive equations for subjective judgments of vertical and horizon in a force field. Acta Otolaryngol (Stockh.) Suppl. 230: 1–20
DiZio P, Lackner JR (1987) The influence of gravitoinertial force level on oculomotor and perceptual responses to sudden stop stimulation. Aviat Space Environ Med 58:A224–A230
DiZio P, Lackner JR (1989) Perceived self-motion elicited by postrotary head tilts in a varying gravitoinertial force background. Percept Psychophys 46:114–118
DiZio P, Lackner JR (1995) Inertial coriolis force perturbations of arm and head movements reveal common, non-vestibular mechanisms. In: Mergner T, Hlavacka F (eds) Multisensory control of posture. Plenum Press, New York, pp 331–338
DiZio P, Lackner JR, Evanoff JN (1987) The influence of gravitoinertial force level on oculomotor and perceptual responses to Coriolis, cross-coupling stimulation. Aviat Space Environ Med 58:A218–A223
Fitzpatrick RC, McCloskey DI (1994) Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol 478:173–186
Glasauer S (1992) Interaction of semicircular canals and otoliths in the processing structure of the subjective zenith. Ann N Y Acad Sci 656:847–849
Gordon CR, Fletcher WA, Melvill Jones G, Block EW (1995) Adaptive plasticity in the control of locomotor trajectory. Exp Brain Res 102:540–545
Grabherr L, Nicoucar K, Mast FW, Merfeld DM (2008) Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res 186:677–681
Graybiel A (1952) Oculogravic illusion. Arch Opthalmol 48:605–615
Graybiel A, Miller EFII, Homick JL (1977) Experiment M131. Human vestibular function. In: Biomedical results from Skylab. US. Government Printing Office, Washington, DC, pp 74–103
Guedry FE Jr, Benson AJ (1978) Coriolis cross-coupling effects: disorienting and nauseogenic or not. Aviat Space Environ Med 49:29–35
Guedry FE Jr, Stockwell CW, Norman JW, Owens GG (1971) Use of triangular waveforms of angular velocity in the study of vestibular function. Acta Otolaryngol 71:439–448
Jurgens R, Boss T, Becker W (1999) Estimation of self-turning in the dark: comparison between active and passive rotation. Exp Brain Res 128:491–504
Kaptein RG, Van Gisbergen JA (2006) Canal and otolith contributions to visual orientation constancy during sinusoidal roll rotation. J Neurophysiol 95:1936–1948
Karmali F, Shelhamer M (2008) The dynamics of parabolic flight: Flight characteristics and passenger percepts. Acta Astronautica 63:594–602
Knierim JJ, McNaughton BL, Poe GR (2000) Three-dimensional spatial selectivity of hippocampal neurons during space flight. Nat Neurosci 3:209–210
Lackner JR, Graybiel A (1985) Head movements elicit motion sickness during exposure to microgravity and macrogravity acceleration levels. In: Igarashi M, Black FO (eds) VII International symposium: vestibular and visual control on posture and locomotor equilibrium. Karger, Basel, pp 170–176
Lackner JR, Ventura J, DiZio P (2006) Dynamic spatial orientation in altered gravitoinertial force environments. Program No. 244.11. In: 2006 neuroscience meeting planner (Online). Society for Neuroscience, Atlanta, GA
Loomis JM, Klatsky RL, Golledge RG, Philbeck JW, Golledge RG (1999) Human navigation by path integration. In: Wayfinding behavior: cognitive mapping and other spatial processes. Johns Hopkins University Press, Baltimore, Maryland, pp 125–151
Merfeld DM (1995) Modeling human vestibular responses during eccentric rotation and off vertical axis rotation. Acta Otolaryngol Suppl 520(Pt 2):354–359
Mergner T, Nardi GL, Becker W, Deecke L (1983) The role of canal-neck interaction for the perception of horizontal trunk and head rotation. Exp Brain Res 49:198–208
Metcalfe T, Gresty MA (1992) Self-controlled reorienting movements in response to rotational displacements in normal subjects and patients with labyrinthine disease. Ann NY Acad Sci 656:695–698
Mittelstaedt ML, Mittelstaedt H (1980) Homing by path integration in mammals. Naturwissenschaften 67:566
O’Keefe J, Nadel L, Keightley S, Kill D (1975) Fornix lesions selectively abolish place learning in the rat. Exp Neurol 48:152–166
Robertson RG, Rolls ET, Georges-Francois P, Panzeri S (1999) Head direction cells in the primate pre-subiculum. Hippocampus 9:206–219
Taube JS (1995) Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci 15:70–86
Taube JS (1998) Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol 55:225–256
Taube JS, Burton HL (1995) Head direction cell activity monitored in a novel environment and during a cue conflict situation. J Neurophysiol 74:1953–1971
Taube JS, Stackman RW, Calton JL, Oman CM (2004) Rat head direction cell responses in zero-gravity parabolic flight. J Neurophysiol 92:2887–2997
van Erp JBF, van Veen H (2006) Touch down: the effect of artificial touch cues on orientation in microgravity. Neurosci Lett 404:78–82
Acknowledgments
Air Force Office of Scientific Research grant F49620-01-10171, NASA grant NAG9-1466.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lackner, J.R., DiZio, P. Angular displacement perception modulated by force background. Exp Brain Res 195, 335–343 (2009). https://doi.org/10.1007/s00221-009-1785-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1785-6