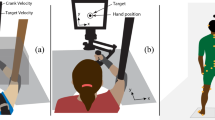
Abstract
When humans explore the external world, hand and arm movements play important roles. Spatio-temporal arrangements of the environment are perceptually generated mainly by means of the sensory–motor integration of the internal model of these movements with the information obtained during the movements. In order to investigate the mechanisms of this integration process, localization tasks have been studied, and previous studies have suggested that the integration process does not work properly around the time of a hand movement. In particular, when a transient vibro-tactile stimulation is presented before, during, or after a hand movement, the stimulus is systematically mislocalized. However, it is debatable whether the tendency to mislocalize a transient stimulus indicates a general failure of the sensory-motor integration process. Here we investigated the generality of the tendency towards mislocalization by observing haptic localizations to different target types, the onset and offset of continuous vibro-tactile stimuli. We found similar types of mislocalizations in responses to the transient vibration and the onset of a continuous vibration, and a clear difference in the types of mislocalizations in responses to the onset and offset of continuous vibrations.



Similar content being viewed by others
References
Azuma R, Haggard P (1999) Effects of response type on coordinated responses during arm movement. Percept Psychophys 61:579–590
Boucher L, Groh JM, Hughes HC (2001) Afferent delays and the mislocalization of perisaccadic stimuli. Vision Res 41:2631–2644
Cai RH, Pouget A, Schlag-Rey M, Schlag J (1997) Perceived geometrical relationships affected by eye-movement signals. Nature 386:601–604
Cordo PJ (1990) Kinesthetic control of a multijoint movement sequence. J Neurophysiol 63:161–172
Cordo PJ, Carlton L, Bevan L, Carlton M, Kerr GK (1994) Proprioceptive coordination of movement sequences: role of velocity and position information. J Neurophysiol 71:1848–1861
Dassonville P (1995) Haptic localization and the internal representation of the hand in space. Exp Brain Res 106:434–448
Dyhre-Poulsen P (1975) Increased vibration threshold before movements in human subjects. Exp Neurol 47:516–522
Flanagan JR, Lolley S (2001) The inertial anisotropy of the arm is accurately predicted during movement planning. J Neurosci 21:1361–1369
Georg K, Hamker FH, Lappe M (2008) Influence of adaptation state and stimulus luminance on peri-saccadic localization. J Vis 8(1):15.1–15.11
Haggard P, Newman CS, Blundell J, Andrew H (2000) The perceived position of the hand in space. Percept Psychophys 62:363–377
Horch KW, Clark FJ, Burgess PR (1975) Awareness of knee joint angle under static conditions. J Neurophysiol 38:1436–1447
Jaskowski P (1996) Simple reaction time and perception of temporal order: dissociations and hypotheses. Percept Mot Skills 82:707–730
Jirsa R, Radil T, Maras L (1992) Perception of timing of movement onset in a simple reaction time task. Neuroreport 3:524–526
Johansson RS, Westling G (1987) Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res 66:141–154
Johnson H, Van Beers RJ, Haggard P (2002) Action and awareness in pointing tasks. Exp Brain Res 146:451–459
Kawato M, Furukawa K, Suzuki R (1987) A hierarchical neural network model for the control and learning of voluntary movements. Biol Cybern 56:1–17
Krakauer JW, Ghilardi MF, Ghez C (1999) Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 2:1026–1031
McCloskey DI, Colebatch JG, Potter EK, Burke D (1983) Judgments about onset of rapid voluntary movements in man. J Neurophysiol 49:851–863
Milne RJ, Aniss AM, Kay NE, Gandevia SC (1988) Reduction in perceived intensity of cutaneous stimuli during movement: a quantitative study. Exp Brain Res 70:569–576
Nijhawan R, Kirschfeld K (2003) Analogous mechanisms compensate for neural delays in the sensory and the motor pathways: evidence from motor flash-lag. Curr Biol 13:749–753
Nishida S, Johnston A (2002) Marker correspondence not processing latency determines temporal binding of visual attributes. Curr Biol 12:359–368
Paillard J, Brouchon M (1968) Active and passive movements in the calibration of position sense. In: Freedman SJ (ed) Neurophysiolosy of spatinally oriented behavior. Dorsey, Homewood, pp 37–55
Post LJ, Zompa IC, Chapman CE (1994) Perception of vibrotactile stimuli during motor activity in human subjects. Exp Brain Res 100:107–120
Sittig AC, Denier van der Gon JJ, Gielen CCAM (1987) The contribution of afferent information on position and velocity demonstrated to the control of slow and fast human forearm movements. Exp Brain Res 67:33–40
Sperry RW (1950) Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43:482–489
Terao M, Watanabe J, Yagi A, Nishida S (2008) Reduction of stimulus visibility compresses apparent time interval. Nat Neurosci 11:541–542
van Beers RJ, Wolpert DM, Haggard P (2001) Sensorimotor integration compensates for visual localization errors during smooth pursuit eye movements. J Neurophysiol 85:1914–1922
von Holst E (1954) Relations between the central nervous system and the peripheral organs. Br J Anim Behav 2:89–94
Watanabe J, Noritake A, Maeda T, Tachi S, Nishida S (2005) Perisaccadic perception of continuous flickers. Vision Res 45:413–430
Wolpert DM, Ghahramani Z, Jordan MI (1995) An internal model for sensorimotor integration. Science 269:1880–1882
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watanabe, J., Nakatani, M., Ando, H. et al. Haptic localizations for onset and offset of vibro-tactile stimuli are dissociated. Exp Brain Res 193, 483–489 (2009). https://doi.org/10.1007/s00221-009-1711-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1711-y