Abstract
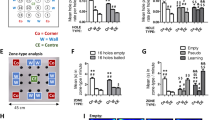
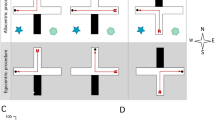
Rats use place (allocentric) or stimulus–response (egocentric) learning strategies for foraging under ethological and/or experimental conditions, proposed to be conveyed by hippocampus or neostriatum, respectively. We investigated here the effect of a reversible blockade of neostriatum on learning strategies assessed by a cross maze paradigm, comparing A × C (phenotypically similar to wild rats) versus Long–Evans rat strains. The rats were trained to reach a consistently baited-arm (west arm), starting from the same arm (south arm). The learning strategy was evaluated at days 11 and 19, when test trials were performed placing the rat in a start-box at the arm (north arm) opposite to that when starting the training, following a saline or lidocaine injection into the neostriatum. Rats entering to the baited-west arm were considered to be place learners and those entering to the unbaited-east arm were response learners. It was found that Long–Evans rats injected with saline were place learners on day 11 and response learners on day 19, but were place learners on both days when injected with lidocaine. A × C rats injected with saline were response learners on days 11 and 19, and place learners on both days when injected with lidocaine. Thus, rat strain influences the memory strategy for solving a cross maze paradigm. Long–Evans, but not A × C rats, shift from place (allocentric) to response (egocentric) learning along the training. When neostriatum was blocked by lidocaine, both rat strains elicited a place learning strategy only.



Similar content being viewed by others
References
Andrews JS (1996) Possible confounding influence of strain, age and gender on cognitive performance in rats. Brain Res Cogn Brain Res 3(3–4):251–267
Andrews JS, Jansen JH, Linders S, Princen A, Broekkamp CL (1995) Performance of four different rat strains in the autoshaping, two-object discrimination, and swim maze tests of learning and memory. Physiol Behav 57(4):785–790
Blodgett HC, McCutchan K (1947) Place versus response learning in the simple T-maze. J Exp Psychol 37:412–422
Blodgett HC, McCutchan K (1948) The relative strength of place and response learning in the T-maze. J Comp Physiol Psychol 41:17–24
Blodgett HC, McCutchan K, Matthews R (1949) Spatial learning in the T-maze: the influence of direction, turn, and food location. J Exp Psychol 39:800–809
Bustos G, Basoalto E, Pinto-Hamuy T (2003) Spatial memory in Long–Evans and Rattus norvegicus rats. Biol Res 36(2):193–199
Clements KM, Saunders AJ, Robertson BA, Wainwright PE (2007) Spontaneously hypertensive, Wistar Kyoto and Sprague–Dawley rats differ in their use of place and response strategies in the water radial arm maze. Neurobiol Learn Mem 87(2):285–294
Colombo PJ, Brightwell JJ, Countryman RA (2003) Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c-Fos in the hippocampus and dorsal striatum. J Neurosci 23(8):3547–3554
Cohen J, Westlake K, Szelest I (2004) Effects of runway shift and stay rules on rats’ serial pattern learning in the T-maze. Learn Behav 32(4):500–511
Dahlin E, Stigsdotter NA, Larsson A, Bäckman L, Nyberg L (2008) Transfer of learning after updating training mediated by the striatum. Science 320:1510–1512
Gleason TC, Dreiling JL, Crawley JN (1999) Rat strain differences in response to galanin on the Morris water task. Neuropeptides 33(4):265–270
Harker KT, Whishaw IQ (2002a) Impaired spatial performance in rats with retrosplenial lesions: importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. J Neurosci 22:1155–1164
Harker KT, Whishaw IQ (2002b) Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fischer 344) and albinism (Wistar, Sprague–Dawley) but not domestication (wild rat vs. Long–Evans, Fischer–Norway). Behav Brain Res 134(1–2):467–477
Harker KT, Whishaw IQ (2004) A reaffirmation of the retrosplenial contribution to rodent navigation: reviewing the influences of lesion, strain and task. Neurosci Biobehav Rev 28(5):485–496
Hill CW, Thune LE (1952) Place and response learning in the white rat under simplified and mutually isolated conditions. J Exp Psychol 43:289–297
Huck UW, Price EO (1975) Differential effects of environmental enrichment on the open-field behavior of wild and domestic Norway rats. J Comp Physiol Psychol 89(8):892–898
Hull CL (1943) Principles of behavior. Appleton-Century Crofts, New York
Lindner MD, Schallert T (1988) Aging and atropine effects on spatial navigation in the Morris water task. Behav Neurosci 102:621–634
Lore R, Flannelly K (1977) Rat societies. Sci Am 236:106–111
McDonald RJ, White NM (1993) A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci 107:3–22
McDonald RJ, White NM (1994) Parallel information processing in the water maze: evidence for independent memory systems involving the dorsal striatum and hippocampus. Behav Neural Biol 61:260–270
Mechan AO, Pm Moran, Elliott M, Young AJ, Joseph MH, Green R (2002) A comparison between Dark Agouti and Sprague–Dawley rats in their behaviour on the elevated plus-maze, open-field apparatus and activity meters, and their response to diazepam. Psychopharmacology 159(2):188–195
Packard MG (1999) Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci USA 96(22):12881–12886
Packard MG, Knowlton BJ (2002) Learning and memory functions of the basal ganglia. Annu Rev Neurosci 25:563–593
Packard MG, McGaugh JL (1992) Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci 106(3):439–446
Packard MG, McGaugh JL (1996) Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 65(1):65–72
Packard MG, White NM (1987) Differential roles of the hippocampus and caudate nucleus in memory: Selective mediation of “cognitive” and “associative” learning. Soc Neurosci Abstr 13:1005
Packard MG, Hirsh R, White NM (1989) Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci 9(5):1465–1472
Pass D, Freeth G (1993) The Rat. ANZCCART News vol 6 no 4 (Insert)
Paxinos G, Watson CH (1986) The rat brain in stereotaxics coordinates. Academic Press, London
Pinto-Hamuy T, Olavarria J, Guic-Robles E, Morgues M, Nassal O, Petit D (1987) Rats with lesions in anteromedial extrastriate cortex fail to learn a visuosomatic conditional response. Behav Brain Res 25:221–231
Prusky GT, Harker KT, Douglas RM, Whishaw IQ (2002) Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav Brain Res 136:339–348
Restle F (1957) Discrimination of cues in mazes: a resolution of the place versus response controversy. Psychol Rev 64:217–228
Saavedra M, Pinto-Hamuy T, Marchant F, Urzúa C (1999) Motivación: su efecto sobre la estrategia de solución del laberinto octogonal. Revista de Psicología de la Universidad de Chile 8 (1): 143–159
Sanchez RF, Montero VM, Espinoza SG, Diaz E, Canitrot M, Pinto-Hamuy T (1997) Visuospatial discrimination deficit in rats after ibotenate lesions in anteromedial visual cortex. Physiol Behav 62(5):989–994
Scharlock DP (1955) The role of extramaze cues in place and response learning. J Exp Psychol 50:249–254
Thompson ME, Thompson JP (1949) Reactive inhibition as a factor in maze learning: II. The role of reactive inhibition in studies of place learning versus response learning. J Exp Psychol 39:883–891
Tolman EC, Ritchie BF, Kalish D (1946) Studies in spatial learning: II. Place versus response learning. J Exp Psychol 36:221–229
Tolman EC, Ritchie BF, Kalish D (1947) Studies in spatial learning: V. Response versus place learning by the noncorrection method. J Exp Psychol 37:285–292
Toth E, Bruin JP, Heinsbroek RP, Joosten RN (1996) Spatial learning and memory in calpastatin-deficient rats. Neurobiol Learn Mem 66:230–235
Van Der Staay FJ, Blokland A (1996) Behavioral differences between outbred Wistar, inbred Fischer 344, brown Norway, and hybrid Fischer 344 × brown Norway rats. Physiol Behav 60(1):97–109
Vorhees CV, Morford LL, Inman SL, Reed TM, Schilling MA, Cappon GD, Moran MS, Nebert DW (1999) Genetic differences in spatial learning between Dark Agouti and Sprague–Dawley strains: possible correlation with the CYP2D2 polymorphism in rats treated neonatally with methamphetamine. Pharmacogenetics 9(2):171–181
Wyss JM, Chambless BD, Kadish I, Van Groen T (2000) Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol Aging 21(5):671–681
Acknowledgments
The work was supported by DID S9608/2; DID SOO-01/2 and FONDECYT 1080447. The excellent technical contribution of Mr. Anibal Martinez is warmly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Espina-Marchant, P., Pinto-Hamuy, T., Bustamante, D. et al. Rat strain influences the use of egocentric learning strategies mediated by neostriatum. Exp Brain Res 193, 205–212 (2009). https://doi.org/10.1007/s00221-008-1610-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1610-7