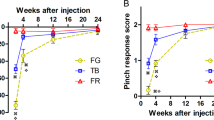
Abstract
Peripheral nerve injury induces the retrograde degeneration of dorsal root ganglion (DRG) cells, which affects predominantly the small-diameter cutaneous afferent neurons. This study compares the time-course of retrograde cell death in cutaneous and muscular DRG cells after peripheral nerve transection as well as neuronal survival and axonal regeneration after primary repair or nerve grafting. For comparison, spinal motoneurons were also included in the study. Sural and medial gastrocnemius DRG neurons were retrogradely labeled with the fluorescent tracers Fast Blue (FB) or Fluoro-Gold (FG) from the homonymous transected nerves. Survival of labeled sural and gastrocnemius DRG cells was assessed at 3 days and 1–24 weeks after axotomy. To evaluate axonal regeneration, the sciatic nerve was transected proximally at 1 week after FB-labeling of the sural and medial gastrocnemius nerves and immediately reconstructed using primary repair or autologous nerve grafting. Twelve weeks later, the fluorescent tracer Fluoro-Ruby (FR) was applied 10 mm distal to the sciatic lesion in order to double-label sural and gastrocnemius neurons that had regenerated across the repair site. Counts of labeled gastrocnemius DRG neurons did not reveal any significant retrograde cell death after nerve transection. In contrast, sural axotomy induced a delayed loss of sural DRG cells, which amounted to 22% at 4 weeks and 43–48% at 8–24 weeks postoperatively. Proximal transection of the sciatic nerve at 1 week after injury to the sural or gastrocnemius nerves neither further increased retrograde DRG degeneration, nor did it affect survival of sural or gastrocnemius motoneurons. Primary repair or peripheral nerve grafting supported regeneration of 53–60% of the spinal motoneurons and 47–49% of the muscular DRG neurons at 13 weeks postoperatively. In the cutaneous DRG neurons, primary repair or peripheral nerve grafting increased survival by 19–30% and promoted regeneration of 46–66% of the cells. The present results suggest that cutaneous DRG neurons are more sensitive to peripheral nerve injury than muscular DRG cells, but that their regenerative capacity does not differ from that of the latter cells. However, the retrograde loss of cutaneous DRG cells taking place despite immediate nerve repair would still limit the recovery of cutaneous sensory functions.


Similar content being viewed by others
References
Akhavan M, Hoang TX, Havton LA (2006) Improved detection of fluorogold-labeled neurons in long-term studies. J Neurosci Methods 152:156–162
Aldskogius H, Kozlova EN (1998) Central neuron–glial and glial–glial interactions following axon injury. Prog Neurobiol 55:1–26
Bennett DLH, French J, Priestley JV, McMahon SB (1996) NGF but not NT-3 or BDNF prevents the A fiber sprouting into lamina II of the spinal cord that occurs following axotomy. Mol Cell Neurosci 8:211–220
Bennett DLH, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV (1998) A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci 18:3059–3072
Bertelli JA, Ghizoni MF (2003) Brachial plexus avulsion injury repairs with nerve transfers and nerve grafts directly implanted into the spinal cord yield partial recovery of shoulder and elbow movements. Neurosurgery 52:1385–1389
Bertelli JA, Taleb M, Saadi A, Mira JC, Pecot-Dechavassine M (1995) The rat brachial plexus and its terminal branches: an experimental model for the study of peripheral nerve regeneration. Microsurgery 16:77–85
Boyd JG, Gordon T (2003) Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol 27:277–323
Carlson J, Lais AC, Dyck PJ (1979) Axonal atrophy from permanent peripheral axotomy in adult cat. J Neuropathol Exp Neurol 38:579–585
Carlstedt T, Cullheim S (2000) Spinal cord motoneuron maintenance, injury and repair. Prog Brain Res 127:501–514
Coggeshall RE, Lekan HA (1996) Methods for determining numbers of cells and synapses: a case for more uniform standarts of review. J Comp Neurol 364:6–15
Coggeshall RE, Kendall G, Woolf CG (1996) Can dorsal root axons survive after being deprived of central and peripheral trophic factors. Soc Neurosci Abstr 759
Curtis R, Tonra JR, Stark JL, Adryan KM, Park JS, Cliffer KD, Lindsay RM, DiStefano PS (1998) Neuronal injury increases retrograde axonal transport of the neurotrophins to spinal sensory neurons and motor neurons via multiple receptor mechanisms. Mol Cell Neurosci 12:105–118
Fu SY, Gordon T (1995) Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci 15:3876–3885
Gillen C, Korfhage C, Müller HW (1997) Gene expression in nerve regeneration. Neuroscientist 3:112–122
Gordon T, Sulaiman O, Boyd JG (2003) Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst 8:236–250
Gu Y, Spasic Z, Wu W (1997) The effects of remaining axons on motoneuron survival and NOS expression following axotomy in the adult rat. Dev Neurosci 19:255–259
Guillery RW (2002) On counting and counting errors. J Comp Neurol 447:1–7
Guillery RW, Herrup K (1997) Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol 386:2–7
Hart AM, Brannstrom T, Wiberg M, Terenghi G (2002) Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat—timecourse of cell death and elimination. Exp Brain Res 142:308–318
Havton L, Kellerth J-O (1987) Regeneration by supernumerary axons with synaptic terminals in spinal motoneurons of cats. Nature 325:711–714
Houle JD, Ye JH (1999) Survival of chronically-injured neurons can be prolonged by treatment with neurotrophic factors. Neuroscience 94:929–936
Hu P, McLachlan EM (2003) Selective reactions of cutaneous and muscle afferent neurons to peripheral nerve transection in rats. J Neurosci 23:10559–10567
Jivan S, Novikova LN, Wiberg M, Novikov LN (2006) The effects of delayed nerve repair on neuronal survival and axonal regeneration after seventh cervical spinal nerve axotomy in adult rats. Exp Brain Res 170:245–254
Karchewski LA, Kim FA, Johnston J, McKnight RM, Verge VMK (1999) Anatomical evidence supporting the potential for modulation by multiple neurotrophins in the majority of adult lumbar sensory neurons. J Comp Neurol 413:327–341
Koliatsos VE, Price WL, Pardo CA, Price DL (1994) Ventral root avulsion: an experimental model of death of adult motor neurons. J Comp Neurol 342:35–44
Kreutzberg GW (1995) Reaction of the neuronal cell body to axonal damage. In: Waxman SG, Kocsis JD, Stys PK (eds) The axon: structure, function and pathophysiology. Oxford University Press, New York, pp 355–374
Kuo LT, Simpson A, Schänzer A, Tse J, An SF, Scaravilli F, Groves MJ (2005) Effects of systemically administered NT-3 on sensory neuron loss and nestin expression following axotomy. J Comp Neurol 482:320–332
Lekan HA, Chung K, Yoon YW, Chung JM, Coggeshall RE (1997) Loss of dorsal root ganglion cells concomitant with dorsal root axon sprouting following segmental nerve lesions. Neuroscience 81:527–534
Li LX, Houenou LJ, Wu WT, Lei M, Prevette DM, Oppenheim RW (1998) Characterization of spinal motoneuron degeneration following different types of peripheral nerve injury in neonatal and adult mice. J Comp Neurol 396:158–168
Ljungberg C, Novikov L, Kellerth JO, Ebendal T, Wiberg M (1999) The neurotrophins NGF and NT-3 reduce sensory neuronal loss in adult rat after peripheral nerve lesion. Neurosci Lett 262:29–32
Lundborg G (2000) A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg [Am] 25:391–414
Lundborg G, Dahlin L, Danielsen N, Zhao Q (1994) Trophism, tropism, and specificity in nerve regeneration. J Reconstr Microsurg 10:345–354
Ma J, Novikov LN, Kellerth JO, Wiberg M (2003) Early nerve repair after injury to the postganglionic plexus: an experimental study of sensory and motor neuronal survival in adult rats. Scand J Plast Reconstr Surg Hand Surg 37:1–9
Ma JJ, Novikov LN, Wiberg M, Kellerth JO (2001) Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: a quantitative morphological study. Exp Brain Res 139:216–223
McKay HA, Brannstrom T, Wiberg M, Terenghi G (2002) Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: timecourse of cell death and elimination. Exp Brain Res 142:308–318
McMahon SB, Armanini MP, Ling LH, Phillips HS (1994) Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron 12:1161–1171
Naumann T, Hartig W, Frotscher M (2000) Retrograde tracing with Fluoro-Gold: different methods of tracer detection at the ultrastructural level and neurodegenerative changes of back-filled neurons in long-term studies. J Neurosci Methods 103:11–21
Novikov L, Novikova L, Kellerth J-O (1995) Brain-derived neurotrophic factor promotes survival and blocks nitric oxide synthase expression in adult rat spinal motoneurons after ventral root avulsion. Neurosci Lett 200:45–48
Novikov L, Novikova L, Kellerth J-O (1997) Brain-derived neurotrophic factor promotes axonal regeneration and long-term survival of adult rat spinal motoneurons in vivo. Neuroscience 79:765–774
Novikova L, Novikov L, Kellerth J-O (1997) Persistent neuronal labeling by retrograde fluorescent tracers: a comparison between Fast Blue, Fluoro-Gold and various dextran conjugates. J Neurosci Methods 74:9–15
Novikova LN, Novikov LN, Kellerth JO (2000) Survival effects of BDNF and NT-3 on axotomized rubrospinal neurons depend on the temporal pattern of neurotrophin administration. Eur J Neurosci 12:776–780
Rhrich-Haddout F, Kassar-Duchossoy L, Bauchet L, Destombes J, Thiesson D, Butler-Browne G, Lyoussi B, Baillet-Derbin C, Horvat JC (2001) Alpha-motoneurons of the injured cervical spinal cord of the adult rat can reinnervate the biceps brachii muscle by regenerating axons through peripheral nerve bridges: combined ultrastructural and retrograde axonal tracing study. J Neurosci Res 64:476–486
Rind HB, Butowt R, Von Bartheld CS (2005) Synaptic targeting of retrogradely transported trophic factors in motoneurons: comparison of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, and cardiotrophin-1 with tetanus toxin. J Neurosci 25:539–549
Smith DH, Chen XH, Pierce JE, Wolf JA, Trojanowski JQ, Graham DI, McIntosh TK (1997) Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma 14:715–727
Snider WD, Elliott JL, Yan Q (1992) Axotomy-induced neuronal death during development. J Neurobiol 23:1231–1246
Stoll G, Muller HW (1999) Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol 9:313–325
Tandrup T (1993) A method for unbiased and efficient estimation of number and mean volume of specified neuron subtypes in rat dorsal root ganglion. J Comp Neurol 329:269–276
Tandrup T, Woolf CJ, Coggeshall RE (2000) Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve. J Comp Neurol 422:172–180
Terenghi G (1999) Peripheral nerve regeneration and neurotrophic factors. J Anat 194:1–14
Terzis JK, Vekris MD, Soucacos PN (1999) Outcomes of brachial plexus reconstruction in 204 patients with devastating paralysis. Plast Reconstr Surg 104:1221–1240
Vanden Noven S, Wallace N, Muccio D, Turtz A, Pinter MJ (1993) Adult spinal motoneurons remain viable despite prolonged absence of functional synaptic contact with muscle. Exp Neurol 123:147–156
Vanderhooft E (2000) Functional outcomes of nerve grafts for the upper and lower extremities. Hand Clin 16:93–104, ix
Vejsada R, Sagot Y, Kato AC (1995) Quantitative comparison of the transient rescue effects of neurotrophic factors on axotomized motoneurons in vivo. Eur J Neurosci 7:108–115
Von Bartheld CS (2001) Comparison of 2-D and 3-D counting: the need for calibration and common sense. Trends Neurosci 24:504–506
West MJ (1999) Stereological methods for estimating the total number of neurons and synapses: issue of precision and bias. Trends Neurosci 22:51–61
Wiberg M, Hazari A, Ljungberg C, Pettersson K, Backman C, Nordh E, Kwast-Rabben O, Terenghi G (2003) Sensory recovery after hand reimplantation: a clinical, morphological, and neurophysiological study in humans. Scand J Plast Reconstr Surg Hand Surg 37:163–173
Wu W, Li L (1993) Inhibition of nitric synthase reduces motoneuron death to spinal root avulsion. Neurosci Lett 153:121–124
Wu WT, Chai H, Zhang JY, Gu HY, Xie YY, Zhou LH (2004) Delayed implantation of a peripheral nerve graft reduces motoneuron survival but does not affect regeneration following spinal root avulsion in adult rats. J Neurotrauma 21:1050–1058
Acknowledgments
This study was supported by the Swedish Medical Research Council (grant number 2005–5892), Umeå University, Hjärnfonden, Åke Wibergs Stiftelse, Anna-Stina och John Mattsons Minnesstiftelse för sonen Johan, Gunvor and Josef Anér’s Foundation and the County of Västerbotten. We thank Mrs. G. Folkesson and Mrs. G. Hällström for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Welin, D., Novikova, L.N., Wiberg, M. et al. Survival and regeneration of cutaneous and muscular afferent neurons after peripheral nerve injury in adult rats. Exp Brain Res 186, 315–323 (2008). https://doi.org/10.1007/s00221-007-1232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-1232-5