Abstract
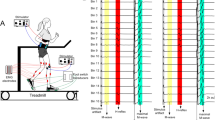
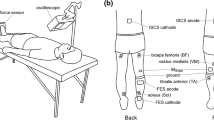
To determine whether the soleus (SOL) H-reflex is modulated during shortening contractions in a manner that has been observed for isometric contractions, SOL H-reflexes and M-waves were elicited via percutaneous electrical stimulation to the tibial nerve at an intensity that evoked an H-reflex at 50% of its maximum in 11 healthy subjects. Paired electrical stimuli were delivered as the ankle angle passed through 90° at an interval of 400 ms while the subject performed shortening contractions at levels of plantar flexion torque ranging between 2 and 30% of that during a maximal voluntary contraction (MVC). H-reflexes were also recorded during the performance of isomeric contractions of plantar flexors at similar levels of plantar flexion torque and at the same joint angle (muscle length) in an additional five healthy subjects. Correlations were examined between the peak-to-peak amplitude of the first H-reflexes, M-waves and plantar flexion torques in both protocols. It was revealed that no significant correlation was found between the SOL H-reflex and increasing plantar flexion torque during shortening contractions (ρ = −0.07, P = 0.15), while a strong positive correlation was observed for the isometric conditions (ρ = 0.99, P < 0.01). No significant change was observed in the SOL M-wave for either contraction type. Furthermore, the H-reflexes elicited via paired stimuli with the same background activity in voluntary shortening contractions showed almost identical amplitudes, suggesting that the level of homosynaptic post-activation depression did not change in response to the varying levels of activation in voluntary shortening contractions. Therefore, the lack of increase in the H-reflex during shortening contractions at increasing intensities is possibly due to a centrally regulated increase in presynaptic inhibition. Such a downward modulation of the reflex suggests that Ia-excitatory input onto the SOL motoneurone pool needs to be reduced during the performance of shortening contractions.





Similar content being viewed by others
References
Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE, Staines WR (1997) Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol 51:393–421
Burke D, Gandevia SC, McKeon B (1984) Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol 52:435–448
Burke D, Adams RW, Skuse NF (1989) The effects of voluntary contraction on the H reflex of human limb muscles. Brain 112(Pt 2):417–433
Butler AJ, Yue G, Darling WG (1993) Variations in soleus H-reflexes as a function of plantarflexion torque in man. Brain Res 632:95–104
Capaday C, Stein RB (1986) Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci 6:1308–1313
Capaday C, Stein RB (1987) Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol 392:513–522
Crone C, Nielsen J (1989) Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res 78:28–32
Crone C, Hultborn H, Jespersen B, Nielsen J (1987) Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol 389:163–185
Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E (1990) Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81:35–45
Floeter MK, Kohn AF (1997) H-reflexes of different sizes exhibit differential sensitivity to low frequency depression. Electroencephalogr Clin Neurophysiol 105:470–475
Guissard N, Duchateau J, Hainaut K (2001) Mechanisms of decreased motoneurone excitation during passive muscle stretching. Exp Brain Res 137:163–169
Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H (1996) On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res 108:450–462
Iles JF (1996) Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol 491(Pt 1):197–207
Jankowska E (1992) Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38:335–378
Kennedy PM, Cresswell AG (2001) The effect of muscle length on motor-unit recruitment during isometric plantar flexion in humans. Exp Brain Res 137:58–64
Koceja DM, Trimble MH, Earles DR (1993) Inhibition of the soleus H-reflex in standing man. Brain Res 629:155–158
Koerber HR, Mendell LM (1991) Modulation of synaptic transmission at Ia-afferent fiber connections on motoneurons during high-frequency stimulation: role of postsynaptic target. J Neurophysiol 65:590–597
Kohn AF, Floeter MK, Hallett M (1997) Presynaptic inhibition compared with homosynaptic depression as an explanation for soleus H-reflex depression in humans. Exp Brain Res 116:375–380
Matthews PB (1986) Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol 374:73–90
Meunier S, Pierrot-Deseilligny E (1989) Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. J Physiol 419:753–763
Meunier S, Pierrot-Deseilligny E (1998) Cortical control of presynaptic inhibition of Ia afferents in humans. Exp Brain Res 119:415–426
Nordlund MM, Thorstensson A, Cresswell AG (2002) Variations in the soleus H-reflex as a function of activation during controlled lengthening and shortening actions. Brain Res 952:301–307
Nordlund MM, Thorstensson A, Cresswell AG (2004) Conditioning Ia-afferent stimulation reduces the soleus Hoffman reflex in humans when muscle spindles are assumed to be inactive. Neurosci Lett 366:250–253
Pierrot-Deseilligny E (1997) Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. J Neurosci Methods 74:189–199
Pierrot-Deseilligny E, Burke D (2005) The circuitry of human spinal cord: its role in motor control and movement disorders. Cambrige University Press, Cambridge
Pinniger GJ, Steele JR, Thorstensson A, Cresswell AG (2000) Tension regulation during lengthening and shortening actions of the human soleus muscle. Eur J Appl Physiol 81:375–383
Pinniger GJ, Nordlund M, Steele JR, Cresswell AG (2001) H-reflex modulation during passive lengthening and shortening of the human triceps surae. J Physiol 534:913–923
Riedo R, Ruegg DG (1988) Origin of the specific H reflex facilitation preceding a voluntary movement in man. J Physiol 397:371–388
Romano C, Schieppati M (1987) Reflex excitability of human soleus motoneurones during voluntary shortening or lengthening contractions. J Physiol 390:271–284
Rudomin P, Schmidt RF (1999) Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129:1–37
Schneider C, Capaday C (2003) Progressive adaptation of soleus H-reflex with daily training at walking backward. J Neurophysiol 89:648–656
Smith AM (1981) The coactivation of antagonist muscles. Can J Physiol Pharmacol 59:733–747
Stein RB, Thompson AK (2006) Muscle reflexes in motion: how, what, and why? Exerc Sport Sci Rev 34:145–153
Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE (2007) Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res 182:309–319
Toft E, Sinkjaer T (1993) H-reflex changes during contractions of the ankle extensors in spastic patients. Acta Neurol Scand 88:327–333
Touge T, Taylor JL, Rothwell JC (1998) Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol 109:489–495
Trimble MH, Du P, Brunt D, Thompson FJ (2000) Modulation of triceps surae H-reflexes as a function of the reflex activation history during standing and stepping. Brain Res 858:274–283
Ugawa Y, Terao Y, Hanajima R, Sakai K, Kanazawa I (1995) Facilitatory effect of tonic voluntary contraction on responses to motor cortex stimulation. Electroencephalogr Clin Neurophysiol 97:451–454
Acknowledgments
The main experiment was supported by funding from the Swedish Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oya, T., Cresswell, A.G. Evidence for reduced efficacy of the Ia-pathway during shortening plantar flexions with increasing effort. Exp Brain Res 185, 699–707 (2008). https://doi.org/10.1007/s00221-007-1198-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-1198-3