Abstract
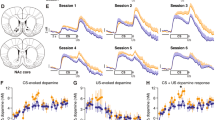
Substantia nigra dopamine neuronal activity in the primate is thought to be related to the error in predicting reward delivery. Dopamine release in rat nucleus accumbens has been shown to increase in relation to drug/food-seeking behaviour. It is not known how the release of dopamine in the striatum corresponds to the many distinct steps of a rewarded, cued task (e.g. recognizing the cue, executing the behaviour, anticipating the reward, receiving the reward) and how dopamine release then changes over time as task performance improves. To investigate dopamine release during a rewarded, cued task and the development of changes in dopamine release over time, changes in extracellular striatal dopamine concentration during a rewarded, cued lever-press task were measured a few days every week for 5 months using high-speed in vivo voltammetry. Rats were trained to press a lever after a tone to obtain a food reward. The reaction time for the lever press decreased gradually as training continued. Changes in dopamine concentration were measured in the anterior striatum (ventral portion) during the task performance after an initial 6-day familiarization period, in which the animals learned that a lever press yielded food, and a 5-week period for surgery, recovery, and electrode preparation. During the task performance, dopamine concentration started to increase just after the cue, peaked near the time of the lever press, and returned to basal levels 1–2 s after the lever press. This pattern of changes in dopamine concentration was observed over the 5 months of testing, the peak dopamine concentration increasing steadily until peaking at week 7, at which time the task performance had not yet improved significantly from week 2. By week 13, task performance had significantly improved and peak dopamine concentration had begun to subside. Thus, the increase in dopamine concentration after the cue was highest while the task was not yet perfected and subsided toward the end of the learning process. It was concluded that striatal dopamine release during a cued lever-press task is relevant to the novelty of the conditions.









Similar content being viewed by others
References
Akiyama A, Kato T, Ishii K, Yasuda E (1985) In vitro measurement of dopamine concentration with carbon fiber electrode. Anal Chem 57:518–522
Benloucif S, Galloway MP (1991) Facilitation of dopamine release in vivo by serotonin agonists: studies with microdialysis. Eur J Pharmacol 200:1–8
Cenci MA, Kalén P, Mandel RJ, Björklund A (1992) Regional differences in the regulation of dopamine and noradrenalin release in medial frontal cortex, nucleus accumbens and caudate-putamen: a microdialysis study in the rat. Brain Res 581:217–228
Cheatwood JL, Reep RL, Corwin JV (2003) The associative striatum: cortical and thalamic projections to the dorsocentral striatum in rats. Brain Res 968:1–14
Church WH, Justice JB, Neill DB (1987) Detecting behaviorally relevant changes in extracellular dopamine with microdialysis. Brain Res 412:397–399
Dewey SL, Smith GS, Logan J, Alexoff D, Ding YS, King P, Pappas N, Brodie JD, Ashby CR Jr (1995) Serotonergic modulation of striatal dopamine measured with positron emission tomography (PET) and in vivo microdialysis. J Neurosci 15:821–829
Fibiger HC, Philips AG, Zis AP (1974) Deficits and instrumental responding after 6-hydroxydopamine lesions of the nigro-neostriatal dopaminergic projection. Pharmacol Biochem Behav 2:87–96
Garside S, Furtado JC, Mazurek MF (1996) Dopamine-glutamate interactions in the striatum: behaviourally relevant modification of excitotoxicity by dopamine receptor-mediated mechanisms. Neuroscience 75:1065–1074
Gerhardt GA, Ksir C, Pivik C, Dickinson SD, Sabeti J, Zahniser NR (1999) Methodology for coupling local application of dopamine and other chemicals with rapid in vivo electrochemical recordings in freely-moving rats. J Neurosci Methods 87:67–76
Hollerman JH, Schultz W (1998) Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci 1:304–309
Ikemoto S, Panksepp J (1999) The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev 31:6–41
Joel D, Weiner I (2000) The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96:451–474
Joseph MH, Dalta K, Young AM (2003) The interpretation of the measurement of nucleus accumbens dopamine by in vivo dialysis: the kick, the craving or the cognition? Neurosci Biobehav Rev 27:527–541
Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM (1992) Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience 51:55–64
Kawagoe R, Takikawa Y, Hikosaka O (1998) Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci 1:411–416
Kiyatkin EA, Gratton A (1994) Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-press for food. Brain Res 652:225–234
Kiyatkin EA, Stein EA (1994) Biphasic changes in mesolimbic dopamine signal during cocaine self-administration. Neurorep 5:1005–1008
Kiyatkin EA, Stein EA (1995) Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an in vivo electrochemical study. Neuroscience 64:599–617
Ljungberg T, Apicella P, Schultz W (1992) Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol 67:145–163
McGinty JF (1999) Regulation of neurotransmitter interactions in the ventral striatum. Ann N Y Acad Sci 877:129–139
Nakazato T, Akiyama A (1988) In vivo voltammetric study of 6-hydroxydopamine-induced neuronal degradation. J Neurochem 51:1007–1013
Nakazato T, Akiyama A (1999) High-speed voltammetry: dual measurement of dopamine and serotonin in vitro. J Neurosci Methods 89:105–110
Nakazato T, Akiyama A (2002) Behavioral activity and stereotypy in rats induced by L-DOPA metabolites: a possible role in the adverse effects of chronicL-DOPA treatment of Parkinson’s disease. Brain Res 930:134–142
Nakazato T, Hosoda S, Akiyama A (1993) A triangular conditioning voltage wave does not influence spontaneous neuronal activity in the rat striatum. J Neurosci Methods 46:69–72
Parent A, Hazrati L-N (1995) Functional anatomy of basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 20:91–127
Radhakishun FS, van Ree JM, Westerink BHC (1988) Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neurosci Lett 85:351–356
Richardson NR, Gratton A (1996) Behavior-relevant changes in nucleus accumbens dopamine transmission elicited by food reinforcement: an electrochemical study in rat. J Neurosci 16:8160–9169
Romo R, Schultz W (1990) Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol 63:592–606
Ross SR, Jackson DM, Wallis EM, Edwards SR (1988) Enhancement by a single dose of reserpine (plus alpha methyl-it p-tyrosine) of the central stimulatory effects evoked by dopamine D-1 and D-2 agonists in the mouse. Naunyn Schmiedebergs Arch Pharmacol 337:512–518
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27
Schultz W (2001) Reward signaling by dopamine neurons. Neurosci 7:293–302
Schultz W, Apicella P, Scarnati E, Ljungberg T (1992) Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 12:4595–4610
Shinba T, Andow Y, Shinozaki T, Ozawa N, Yamamoto K (1998) Phasic increase of monoamine-related electrochemical signal in the rat caudate nucleus following conditioned auditory stimulation during the reaction-time task. Brain Res 781:284–290
Stamford JA, Kruk ZL, Palij P, Millar J (1988) Diffusion and uptake of dopamine in rat caudate and nucleus accumbens compared using fast cyclic voltammetry. Brain Res 448:381–385
Swanson LW (1998) Brain maps: structure of the rat brain. Elsevier, Amsterdam
Toner CC, Stamford JA (1996) “Real time” measurement of dopamine release in an in vitro model of neostriatal ischemia. J Neurosci Methods 67:133–140
Walker QD, Rooney MB, Wightman RM, Kuhn CM (2000) Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 95:1061–1070
Young SD, Michael AC (1993) Voltammetry of extracellular dopamine in rat striatum during ICSS-like electrical stimulation of the medial forebrain bundle. Brain Res 600:305–307
Young AM, Joseph MH, Gray JA (1992) Increased dopamine release in vivo in nucleus accumbens and caudate nucleus of the rat during drinking: a microdialysis study. Neuroscience 48:871–876
Acknowledgements
This study was supported in part by a Grant-in-Aid (No. 12680782) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. I express my gratitude to Dr. Akitane Akiyama of the Laboratory of Rainbow Science in Yokohama, Japan. These studies could not have been accomplished without his technical support and advice. I thank the former Professor Okihide Hikosaka (Laboratory of Sensorimotor Research, National Eye Institute, National Institute of Health, Bethesda, USA) and Dr. Yoriko Takikawa (Department of Physiology, Juntendo University School of Medicine) for their helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakazato, T. Striatal dopamine release in the rat during a cued lever-press task for food reward and the development of changes over time measured using high-speed voltammetry. Exp Brain Res 166, 137–146 (2005). https://doi.org/10.1007/s00221-005-2345-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-2345-3