Abstract
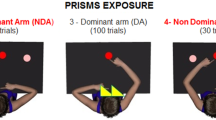
Prism adaptation is a useful paradigm to study the integration and reorganization of various sensory modalities involved in sensory–motor tasks. By prolonging the prismatic aftereffect and well-timed observation, we aimed to dissociate the components and mechanisms involved in human prism adaptation by their differential decay and development time courses. Here, we show that a single session of prism adaptation training, combining small increments of prism strength below the subjects’ awareness threshold, during a pointing task with a free walk session with total prism exposure duration of 75 min, generated a surprisingly long-lasting aftereffect. The aftereffect was measured by the magnitude of the proprioceptive shift (assessed by straight-ahead pointing in the dark) for 7 days. An aftereffect was observed, which lasted for more than 6 days, by a single prism adaptation session. The aftereffect did not decay gradually. Unlike previous descriptions, the aftereffect showed two separate time-courses of decay and increase. After a significant initial decay within 6 h, the aftereffect increased again from 1 day up to 3 days. The novel decay and delayed development profile of this adaptation aftereffect suggests two separate underlying neural mechanisms with different time scales. Our experimental paradigms promise to reveal directly the temporal characteristics of early versus late long-term neural plasticity in complex human adaptive behavior.


Similar content being viewed by others
References
Baizer JS, Kralj-Hans I, Glickstein M (1999) Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol 81:1960–1965
Boyden ES, Katoh A, Raymond JL (2004) Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci 27:581–609
Butler AJ, Fink GR, Dohle C, Wunderlich G, Tellmann L, Seitz RJ, Zilles K, Freund HJ (2004) Neural mechanisms underlying reaching for remembered targets cued kinesthetically or visually in left or right hemispace. Hum Brain Mapp 21:165–177
Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR (2004) Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci 24:8662–8671
Calabria M, Michel C, Honoré J, Guillaume A, Pisella L, Luauté, Rode G, Boisson D, Rossetti Y (2004) Prism adaptation and the lack of awareness in spatial neglect. European Congress of Neuropsychology, 18–20 April, Modena
Choe CS, Welch RB (1974) Variables affecting the intermanual transfer and decay of prism adaptation. J Exp Pshychol 102:1076–1084
Chklovskii DB, Mel BW, Svoboda K (2004) Cortical rewiring and information storage. Nature 431:782–788
Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP, Alexander GE (1996) Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature 383:618–621
Clower DM, West RA, Lynch JC, Strick PL (2001) The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci 21:6283–6291
Clower DM, Dum RP, Strick PL (2005) Basal ganglia and cerebellar inputs to ‘AIP’. Cereb Cortex 15:913–920 (Epub 2004 Sep 30)
Colent C, Pisella L, Bernieri C, Rode G, Rossetti Y (2000) Cognitive bias induced by visuo-motor adaptation to prisms: a simulation of unilateral neglect in normal individuals? Neuroreport 11:1899–1902
Farne A, Rossetti Y, Toniolo S, Ladavas E (2002) Ameliorating neglect with prism adaptation: visuo-manual and visuo-verbal measures. Neuropsychologia 40:718–729
Goedert KM, Willingham DB (2002) Patterns of interference in sequence learning and prism adaptation inconsistent with the consolidation hypothesis. Learn Mem 9:279–292
Goldberg, Taub E, Berman AJ, et al (1967) Decay of prism after-effect and interlimb transfer of adaptation. Paper presented at the meeting of Eastern Psychological Association, Boston, April
Hamilton CR, Bossom J (1964) Decay of prism aftereffects. J Exp Psychol 67:148–150
Hatada Y, Rossetti Y (2004a) Long-lasting prism-adaptation aftereffects: Shift in open-loop midsagittal pointing involves more than just visual and proprioceptive components. Perception 33(Suppl):140
Hatada Y, Rossetti Y (2004b) Prism adaptation generates a very long-lasting directionally biased proprioceptive shift in healthy subjects. Soc Neurosci Abstr 524.12
Hatada Y, Wu F, Silverman R, Schacher S, Goldberg DJ (1999) En passant synaptic varicosities form directly from growth cones by transient cessation of growth cone advance but not of actin-based motility. J Neurobiol 41:242–251
Hatada Y, Wu F, Sun ZY, Schacher S, Goldberg DJ (2000) Presynaptic morphological changes associated with long-term synaptic facilitation are triggered by actin polymerization at preexisting varicosities. J Neurosci 20:RC82
Hay J, Pick HL Jr (1966) Visual and proprioceptive adaptation to optical displacement of the visual stimulus. J Exp Psychol 71:150–158
Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M (2000) Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403:192–195
Ingram H, van Donkelaar P, Cole J, Vercher JL, Gauthier G, Miall RC (2000) The role of proprioception and attention in a visuomotor adaptation task. Exp Brain Res 132:114–126
Ito M (2001) Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev 81:1143–1195
Jakobson LS, Goodale MA (1989) Trajectories of reaches to prismatically-displaced targets: evidence for “automatic” visuomotor recalibration. Exp Brain Res 78:575–587
Kagerer FA, Contreras-Vidal JL, Stelmach GE (1997) Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115(3):557–561
Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038
Klapp ST, Nordell SA, Hoekenga KC, Patton CB (1974) Long-lasting aftereffect of brief prism exposure. Percept Psychophys 15:399–400
Kurata K, Hoshi E (1999) Reacquisition deficits in prism adaptation after muscimol microinjection into the ventral premotor cortex of monkeys. J Neurophysiol 81:1927–1938
Lackner JR, Lobovits D (1977) Adaptation to displaced vision: evidence for prolonged after-effects. Q J Exp Psychol 29(1):65–69
Martin KC, Kosik KS (2002) Synaptic tagging—who’s it? Nat Rev Neurosci 3:813–820
Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT (1996) Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119:1183–1198
Maviel T, Durkin TP, Menzaghi F, Bontempi B (2004) Sites of neocortical reorganization critical for remote spatial memory. Science 305:96–99
Michel C (2003) Les effets consécutifs cognitifs de l’adaptation prismatique visuo-manuelle: de la pseudonégligence à la négligence. Unpublished PhD thesis, Université Claude Bernard Lyon, Villeurbanne
Oblinger MM, Lasek RJ (1985) Selective regulation of two axonal cytoskeletal networks in dorsal root ganglion cells. In: O’Lague P (ed) UCLA Symposium on Molecular and Cellular Biology, vol 24, New York, pp135–143
Pisella L, Rode G, Farne A, Boisson D, Rossetti Y (2002) Dissociated long lasting improvements of straight-ahead pointing and line bisection tasks in two hemineglect patients. Neuropsychologia 40:327–334
Pisella L, Rossetti Y, Michel C, Rode G, Boisson D, Pelisson D, Tilikete C (2005) Ipsidirectional impairment of prism adaptation after unilateral lesion of anterior cerebellum. Neurology 65:150–152
Redding GM, Wallace B (1985) Perceptual-motor coordination and adaptation during locomotion: determinants of prism adaptation in hall exposure. Percept Psychophys 38:320–330
Redding GM, Wallace B (1996) Adaptive spatial alignment and strategic perceptual-motor control. J Exp Psychol Hum Percept Perform 22:379–394
Redding GM, Wallace B (1997a) Prism adaptation during target pointing from visible and nonvisible starting locations. J Mot Behav 29:119–130
Redding GM, Wallace B (1997b) Adaptive spatial alignment. Lawrence Erlbaum, Hillsdale, NJ
Redding GM, Wallace B (2002) Strategic calibration and spatial alignment: a model from prism adaptation. J Mot Behav 34:126–138
Redding GM, Wallace B (2003) Dual prism adaptation: calibration or alignment? J Mot Behav 35(4):399–408
Redding GM, Wallace B (2005) Prism adaptation and unilateral neglect: review and analysis. Neuropsychologia (in press) (Epub ahead of print)
Redding GM, Rossetti Y, Wallace B (2005) Applications of prism adaptation: a tutorial in theory and method. Neurosci Biobehav Rev 29:431–444
Robertson EM, Pascual-Leone A, Press DZ (2004) Awareness modifies the skill-learning benefits of sleep. Curr Biol 14:208–212
Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, Perenin MT (1998) Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 395:166–169
Sutton MA, Masters SE, Bagnall MW, Carew TJ (2001) Molecular mechanisms underlying a unique intermediate phase of memory in aplysia. Neuron 31:143–154
Takehara K, Kawahara S, Kirino Y (2003) Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 23:9897–9905
Taub E, Goldberg LA (1973) Prism adaptation: control of intermanual transfer by distribution of practice. Science 180:755–757
Taub E, Ellman SJ, Berman AJ (1966) Deafferentation in monkeys: effect on conditioned grasp response. Science 151:593–594
Walker MP, Stickgold R (2004) Sleep-dependent learning and memory consolidation. Neuron 44:121–133
Welch RB (1978) Perceptual modification: adaptating to altered sensory environments. Academic, New York, NY
Welch RB (1986) Adaptation of space perception. In Boff KR, Kaufman L, Thomas JR (eds) Handbook of perception and human performance, vol 1: sensory processes and perception. Wiley, Chichester, NY, pp24.1–24.45
Weiner MJ, Hallett M, Funkenstein HH (1983) Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology 33:766–772
Yin PB, Kitazawa S (2001) Long-lasting aftereffects of prism adaptation in the monkey. Exp Brain Res 141:250–253
Acknowledgements
The authors wish to thank the subjects who participated for this experiment for their patience; A. Koene, S. Lin, C. McManus, C. Michel, L. Pisella, G. Redding, P. Salin for their suggestions and constructive criticisms and P. Revol, C. Urquizar for their technical help. This work was supported by funds from INSERM PROGRES to YR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hatada, Y., Miall, R. & Rossetti, Y. Two waves of a long-lasting aftereffect of prism adaptation measured over 7 days. Exp Brain Res 169, 417–426 (2006). https://doi.org/10.1007/s00221-005-0159-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0159-y