Abstract
In humans, the flexor carpi radialis (FCR) and extensor carpi radialis (ECR) muscles act as antagonists during wrist flexion-extension and as functional synergists during radial deviation. In contrast to the situation in most antagonist muscle pairs, Renshaw cells innervated by the motor neurons of each muscle inhibit the motoneurons, but not Ia inhibitory interneurons, of the opposite motor pool. Here we compared gain regulation of spinal circuits projecting to FCR motoneurons during two tasks: flexion and radial deviation of the wrist. We also investigated the functional consequences of this organisation for maximal voluntary contractions (MVCs). Electromyographic (EMG) recordings were taken from FCR, ECR longus and ECR brevis using fine-wire electrodes and electrical stimulation was delivered to the median and radial nerves. Ten volunteers participated in three experiments.
-
1.
To study the regulation of the Renshaw cell-mediated, inhibitory pathway from ECR to FCR motoneurons, forty stimuli were delivered to the radial nerve at 50% of the maximal M-wave amplitude for ECR brevis. Stimuli were delivered during both isometric wrist flexions and radial deviation actions with an equivalent EMG amplitude in FCR (~5% wrist flexion MVC).
-
2.
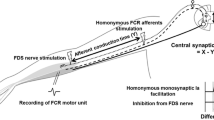
To explore the homonymous Ia afferent pathway to FCR motoneurons, 50 stimuli were delivered to the median nerve at intensities ranging from below motor threshold to at least two times that which evoked a maximal M-wave during wrist flexion and radial deviation (matched FCR EMG at ~5% wrist flexion MVC).
-
3.
EMG amplitude was measured during MVCs in wrist flexion, extension and radial deviation.
There was no significant difference in the inhibition of FCR EMG induced via ECR-coupled Renshaw cells between radial deviation and wrist flexion. However, the mean FCR H-reflex amplitude was significantly (P<0.05) greater during wrist flexion than radial deviation. Furthermore, EMG amplitude in FCR and ECR brevis was significantly (P<0.05) greater during MVCs in wrist flexion and extension (respectively) than radial deviation. ECR longus EMG was significantly greater during MVCs in radial deviation than extension. These results indicate that the gain of the Renshaw-mediated inhibitory pathway between ECR and FCR motoneurons is similar for weak flexion and radial deviation actions. However, the gain of the H-reflex pathway to FCR is greater during wrist flexion than radial deviation. Transmission through both of these pathways probably contributes to the inability of individuals to maximally activate FCR during radial deviation MVCs.




Similar content being viewed by others
References
Aimonetti JM, Vedel JP, Schmied A, Pagni S (2001) Changes in the tonic activity of wrist extensor motor units induced by stimulating antagonistic group 1 afferents in humans. Exp Brain Res 141:21–32
Aimonetti JM, Vedel JP, Schmied A, Pagni S (2000a) Inhibition versus facilitation of the reflex responsiveness of identified wrist extensor motor units by antagonist flexor afferent inputs in humans. Exp Brain Res 133:391–401
Aimonetti JM, Vedel JP, Schmied A, Pagni S (2000b) Task dependence of Ia presynaptic inhibition in human wrist extensor muscles: a single motor unit study. Clin Neurophysiol 111:1165–1174
Aymard C, Baret M, Katz R, Lafitte C, Penicaud A, Raoul S (2001) Modulation of presynaptic inhibition of la afferents during voluntary wrist flexion and extension in man. Exp Brain Res 137:127–131
Aymard C, Chia L, Katz R, Lafitte C, Penicaud A (1995) Reciprocal inhibition between wrist flexors and extensors in man: a new set of interneurones? J Physiol 487:221–235
Aymard C, Decchi B, Katz R, Lafitte C, Penicaud A, Raoul S, Rossi A (1997) Recurrent inhibition between motor nuclei innervating opposing wrist muscles in the human upper limb. J Physiol 499:267–282
Burke D, Gandevia SC, McKeon B (1984) Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol 52:435–448
Baret M, Katz R, Lamy JC, Penicaud A, Wargon I (2003) Evidence for recurrent inhibition of reciprocal inhibition from soleus to tibialis anterior in man. Exp Brain Res 152:133–136
Carroll TJ, Baldwin ERL (2004) Task dependence of muscle activity and reflex function in human wrist flexors and extensors. Proc Aust Neurosci Soc Meeting, p 102
Hultborn H, Jankowska E, Lindstrom S (1971a) Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol 215:591–612
Hultborn H, Jankowska E, Lindstrom S (1971b) Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol 215:613–636
Hultborn H, Jankowska E, Lindstrom S (1971c) Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol 215:637–664
Illert M, Wietelmann D (1989) Distribution of recurrent inhibition in the cat forelimb. In: Allum JHL, Hulliger M (eds) Progress in brain research: afferent control of posture and locomotion, vol 80, pp 273–281. Elsevier, Amsterdam
Marchand-Pauvert V, Nicolas G, Burke D, Pierrot-Deseilligny E (2002) Suppression of the H-reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley. J Physiol 542:963–976
Misiaszek JE (2003) The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 28:144–160
Nielsen J, Kagamihara Y (1992) The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. J Physiol 456:373–391
Nielsen J, Kagamihara Y (1993) The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol 464:575–593
Nielsen J, Pierrot-Deseilligny E (1996) Evidence of facilitation of soleus-coupled Renshaw cells during voluntary co-contraction of antagonistic ankle muscles in man. J Physiol 49:603–611
Nielsen J, Sinkjaer T, Toft E, Kagamihara Y (1994) Segmental reflexes and ankle joint stiffness during co-contraction of antagonistic ankle muscles in man. Exp Brain Res 102:350–358
Riek S, Carson RG, Wright A (2000) A new technique for the selective recording of extensor carpi radialis longus and brevis EMG. J Electromyogr Kinesiol 10:249–253
Rossi A, Decchi B, Zalaffi A, Mazzocchio R (1995) Group 1a non-reciprocal inhibition from wrist extensor to flexor motoneurones in humans. Neurosci Lett 191:205–207
Tyler AE, Hutton RS (1986) Was Sherrington right about co-contractions? Brain Res 370:171–175
Acknowledgements
We thank Alejandro Ley for assistance with data collection and Zoltan Kenwell for technical assistance. The work was supported by the Alberta Heritage Foundation for Medial Research (DC) and the Killam Trust (TC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carroll, T.J., Baldwin, E.R.L. & Collins, D.F. Task dependent gain regulation of spinal circuits projecting to the human flexor carpi radialis. Exp Brain Res 161, 299–306 (2005). https://doi.org/10.1007/s00221-004-2072-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2072-1