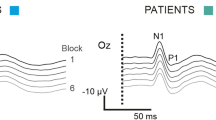
Abstract.
In a previous comparative study with migraineurs, we found in 24 normal subjects that the amplitude of the pattern-reversal visual evoked potential (PR-VEP) in the first block of 100 responses and its habituation over 6 sequential blocks were significantly decreased after 1 Hz repetitive transcranial magnetic stimulation (rTMS), while 10 Hz rTMS had no significant effect. We report here our results on the reproducibility of the rTMS effect studied in ten of these subjects by repeating the recordings for each frequency three times on different days. We have also reanalysed the data obtained in 24 normal subjects, looking separately at the results in those stimulated at an intensity equal to phosphene threshold (group 1; n=14) and those stimulated at 110% of motor threshold because of unelicitable phosphenes (group 2; n=10). We finally determined the precise duration of the rTMS effect. Despite some interindividual variability, the effects of both rTMS frequencies on first block amplitude, habituation between first and sixth block and habituation slope over the six blocks were highly reproducible. The only difference between the two groups of subjects was the effect of 1 Hz rTMS on the second measured PR-VEP component. Whereas first block amplitude of the first P1-N1 component and habituation were decreased in both groups, such a decrease was found for the second P1-N2 component only in group 1 stimulated at phosphene threshold. The dishabituation of the N1-P1 component after 1 Hz rTMS was maximal at 15 min, but lasted up to 33 min, while that of P1-N2 disappeared after 3 min. There was a non-significant trend (p=0.06) for a reduction of first block amplitude after 10 Hz rTMS in the total group of subjects, but no effect on habituation. The inhibitory effect of 1 Hz rTMS, which reduces in healthy controls both first block PR-VEP amplitude and habituation, probably by decreasing the preactivation excitability level of the underlying visual cortex, is thus reproducible and long lasting. Long trains of 10 Hz rTMS tend to attenuate reproducibly the cortical preactivation level in normal subjects, but they do not affect habituation at all, which contrasts with their effect in migraineurs, in whom, as previously reported, they significantly correct the habituation deficit. The absence of an effect of 1 Hz rTMS on PR-VEP P1-N2 in subjects stimulated at 110% of motor threshold may be explained by the deeper anatomical location of the cortical generators of this component and the lower stimulation intensity used. Taken together our results confirm that the effect of rTMS on the underlying cortex depends on several variables such as frequency, intensity and level of cortical preactivation.



Similar content being viewed by others
References
Áfra J, Cecchini AP, De Pasqua V, Albert A, Schoenen J (1998a) Visual evoked potentials during long periods of pattern-reversal stimulation in migraine. Brain 121:233–241
Áfra J, Mascia A, Gerard P, Maertens de Noordhout A, Schoenen J (1998b) Interictal cortical excitability in migraine: a study using transcranial magnetic stimulation of motor and visual cortices. Ann Neurol 44:209–215
Aurora SK, Ahmad BK, Welch KM, Bhardhwaj P, Ramadan NM (1998) Transcranial magnetic stimulation confirms hyperexcitability of occipital cortex in migraine. Neurology 50:1111–1114
Bogacz P, Vanzulli A, Handler D, Garcia Austt E (1970) Evoked potentials in man. II. Habituation of visual evoked responses. Acta Neurol Latinoamericana 3:353–362
Bohotin V, Fumal A, Vandenheede M, Gerard P, Bohotin C, Maertens de Noordhout A, Schoenen J (2002) Effects of repetitive transcranial magnetic stimulation on visual evoked potentials in migraine. Brain 125:912–922
Boroojerdi B, Prager A, Muellbacher W, Cohen LG (2000a) Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology 54:1529–1531
Boroojerdi B, Bushara KO, Corwell B, Immisch I, Battaglia F, Muellbacher W, Cohen LG (2000b) Enhanced excitability of the human visual cortex induced by short-term light deprivation. Cereb Cortex 10:529–534
Brandt SA, Brocke J, Röricht S, Ploner CJ, Villringer A, Meyer BU (2001) In vivo assessment of human visual system connectivity with transcranial electrical stimulation during functional magnetic resonance imaging. Neuroimage 14:366–375
Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG (1997a) Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48:1398–1403
Chen R, Gerloff C, Classen J, Wassermann EM, Hallet M, Cohen LG (1997b) Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 105:415–421
Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA (2002) Cortical sources of the early components of the visual evoked potentials. Hum Brain Mapp 15:95–111
Enomoto H, Ugawa Y, Hanajima R, Yuasa K, Mochizuki H, Terao Y, Shiio Y, Furubayashi T, Iwata NK, Kanazawa I (2001) Decreased sensory cortical excitability after 1 Hz rTMS over the ipsilateral primary motor cortex. Clin Neurophysiol 112:2154–2158
Fierro B, Piazza A, Brighina F, La Bua V, Buffa D, Oliveri M (2001) Modulation of intracortical inhibition induced by low- and high-frequency repetitive transcranial magnetic stimulation. Exp Brain Res 138:452–457
Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, Martin C, Jerabek P (1997) Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport 8:2787–2791
Gerschlager W, Siebner HR, Rothwell JC (2001) Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology 57:449–55
Hamilton RH, Pascual-Leone A (1998) Cortical plasticity associated with Braille learning. Trends Cogn Sci 2:168–174
Kandel ER (1992) Cellular mechanisms of learning and biological basis of individuality. In: Kandel ER, Schwartz JH, Jessell TM (eds) Principles of neurol science. Elsevier, New York, 1009–1031
Knott JR, Irwin DA (1973) Anxiety, stress and the contingent negative variation. Arch Gen Psychiatry 29:538–541
Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM (1999) The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science 284:167–170
Maeda F, Keenan J, Tormos JM, Topka H, Pascual-Leone A (2000) Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 111:800–805
Megela AL, Teyler TJ (1979) Habituation of the human evoked potential. J Comp Physiol Psychol 93:1154–1170
Mesulam MM (1990) Large-scale neurocognitive networks and distributed processing for attention language and memory. Ann Neurol 28:597–613
Modugno N, Nakamura Y, MacKinnon CD, Filipovic SR, Bestman S, Berardelli A, Rothwell JC (2001) Motor cortex excitability following short trains of repetitive magnetic stimuli. Exp Brain Res 140:453–459
Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallet M (1994) Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117:847–858
Paus T, Castro-Alamancos MA, Petrides M (2001) Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci 14:1405–1411
Peinemann A, Lehner C, Mentschel C, Münchau A, Conrad B, Siebner HR (2000) Subthreshold 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett 296:21–24
Romero JF, Anschel D, Sparing R, Gangitano M, Pascual-Leone P (2002) Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol 113:101–107
Sándor PS, Áfra J, Proietti-Cecchini A, Albert A, Schoenen J (1999) Familial influences on cortical evoked potentials in migraine. Neuroreport 10:1235–1238
Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW (1992) Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab 12:584–592
Schoenen J (1996) Deficient habituation of evoked cortical potentials in migraine: a link between brain biology, behavior and trigeminovascular activation? Biomed Pharmacother 50:71–78
Schoenen J, Wang W, Albert A, Delwaide PJ (1995) Potentiation instead of habituation characterizes visual evoked potentials in migraine patients between attacks. Eur J Neurol 2:115–122
Shigeto H, Tobimatsu S, Yamamoto T, Kobayashi T, Kato M (1998) Visual evoked cortical magnetic responses to checkerboard pattern reversal stimulation: a study on the neural generators of N75, P100 and N145. J Neurol Sci 156:186–194
Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM (1999) Menstrual cycle effects on cortical excitability. Neurology 53:2069–2072
Speer AM, Kimbrell TA, Wassermann EMD, Repella J, Willis MW, Herscovitch P, Post RM (2000) Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 48:1133–1141
Wu T, Sommer M, Tergau F, Paulus W (2000) Lasting influence of repetitive transcranial magnetic stimulation on intracortical excitability in human subjects. Neurosci Lett 287:37–40
Acknowledgements.
This study was supported by grant no. 3.4523.00 from the Belgian Fund for Medical Research (Brussels) and grant no. 125 from the Migraine Trust (London) to J.S. V.B. is the recipient of a Clinical Fellowship of the International Headache Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Fumal and V. Bohotin contributed equally to this study
An erratum to this article is available at http://dx.doi.org/10.1007/s00221-003-1533-2.
Rights and permissions
About this article
Cite this article
Fumal, A., Bohotin, V., Vandenheede, M. et al. Effects of repetitive transcranial magnetic stimulation on visual evoked potentials: new insights in healthy subjects. Exp Brain Res 150, 332–340 (2003). https://doi.org/10.1007/s00221-003-1423-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1423-7