Abstract.
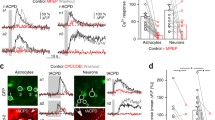
Fluoroacetate is known to block cell metabolism and to change potassium conductances selectively in astrocytes. In a functional neuronal network with ongoing activity, we investigated the effects of such a blockade of the astrocytic metabolism by fluoroacetate on neuronal signal propagation. Transverse 400-µm slices were prepared from the caudal medulla of mice of postnatal day 3–8, which contained the hypoglossal nucleus receiving excitatory synaptic input from the ventral respiratory group. Propagation of excitation within this network was measured by optical imaging using the voltage-sensitive dye RH 795. A 464-element photodiode array allowed fast recordings of voltage changes within a small population of cells. The spatial and temporal resolution was advanced to 32 µm and 1.27 ms, respectively. Changes of cellular membrane potential levels were expressed as relative changes of fluorescence (ΔI/I). Stimulus-evoked excitation of neurons propagating from the ventral respiratory group to the hypoglossal nucleus peaked after 7.2±0.6 ms (n=6). The latency of this early excitatory response is consistent with the time course of stimulus-evoked EPSPs in whole-cell recordings. Mean changes of fluorescence in the hypoglossal nucleus were −2.1±0.5×10−3 (ΔI/I). After incubation in 1 mM fluoroacetate, the early depolarization was reduced to 69.1±9.8% of control (n=6, p=0.034). Additionally, fluoroacetate induced a delayed excitatory response, such that fluorescence intensity did not return to baseline within 1 s. Propagation velocity and spatial distribution of the voltage signal were not affected by fluoroacetate. Our results suggest that blockade of astrocyte metabolism impairs fast synaptic transmission and induces a delayed excitation, probably resulting from the combination of reduced repolarization of neurons and persistent depolarization of astrocytes.


Similar content being viewed by others
References
Albowitz B, Kuhnt U, Köhling R, Lücke A, Straub H, Speckmann E-J, Tuxhorn I, Wolf P, Pannek H, Oppel F (1998) Spatio-temporal distribution of epileptiform activity in slices from human neocortex: recordings with voltage-sensitive dyes. Epilepsy Res 32:224–232
Barish ME, Ichikawa M, Tominaga T, Matsumoto G, Iijima T (1996) Enhanced fast synaptic transmission and a delayed depolarization induced by transient potassium current blockade in rat hippocampal slice as studied by optical recording. J Neurosci 16:5672–5687
Davila HV, Cohen LB, Salzberg BM, Shrivastav BB (1974) Changes in ANS and TNS fluorescence in giant axons from Loligo. J Membr Biol 15:29–46
Fonnum F, Johnsen A, Hassel B (1997) Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia 21:106–113
Gardner-Medwin AR, Coles JA, Tsacopoulos M (1981) Clearance of extracellular potassium: evidence for spatial buffering by glial cells in the retina of the drone. Brain Res 209:452–457
Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U (1997) Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J Cereb Blood Flow Metab 17:1230–1238
Hülsmann S, Oku Y, Zhang W, Richter DW (2000a) Metabolic coupling between glia and neurons is necessary for maintaining respiratory activity in transverse medullary slices of neonatal mouse. Eur J Neurosci 12:856–862
Hülsmann S, Oku Y, Zhang W, Richter DW (2000b) Metabotropic glutamate receptors and blockade of glial Krebs cycle depress glycinergic synaptic currents of mouse hypoglossal motoneurons. Eur J Neurosci 12:239–246
Keyser DO, Pellmar TC (1994) Synaptic transmission in the hippocampus: critical role for glial cells. Glia 10:237–243
Köhling R, Höhling JM, Straub H, Kuhlmann D, Kuhnt U, Tuxhorn I, Ebner A, Wolf P, Pannek HW, Gorji A, Speckmann EJ (2000) Optical monitoring of neuronal activity during spontaneous sharp waves in chronically epileptic human neocortical tissue. J Neurophysiol 84:2161–2165
Köhling R, Reinel J, Vahrenhold R, Hinrichs K, Speckmann E-J (2002) Spatio-temporal patterns of neuronal activity: analysis of optical imaging data using geometric shape matching. J Neurosci Methods 114:17–23
Largo C, Ibarz JM, Herreras O (1997) Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J Neurophysiol 78:295–307
Magistretti PJ, Pellerin L, Rothman DL, Shulman RG (1999) Energy on demand. Science 283:496–497
Martinez-Hernandez A, Bell KP, Norenberg MD (1977) Glutamine synthetase: glial localization in brain. Science 195:1356–1358
Oku Y, Hülsmann S, Zhang W, Richter DW (1999) Modulation of glycinergic synaptic current kinetics by octanol in mouse hypoglossal motoneurons. Pflügers Arch 438:656–664
Reigart JR, Brueggeman, Keil JE (1975) Sodium fluoroacetate poisoning. Am J Dis Child 129:1224–1226
Richter DW, Spyer KM (2001) Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci 24:464–742
Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA (1997) Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature 385:630–634
Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL (1991) Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254:726–729
Sonnewald U, Westergaard N, Schousboe A (1997) Glutamate transport and metabolism in astrocytes. Glia 21:56–63
Swanson RA, Graham SH (1994) Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res 664:94–100
Szerb JC, Issekutz B (1987) Increase in the stimulation-induced overflow of glutamate by fluoroacetate, a selective inhibitor of the glial tricarboxylic cycle. Brain Res 410:116–120
Acknowledgements.
The work was supported by the Deutsche Forschungsgemeinschaft. The authors are grateful to Mrs. Herrenpoth for the excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hülsmann, S., Straub, H., Richter, D.W. et al. Blockade of astrocyte metabolism causes delayed excitation as revealed by voltage-sensitive dyes in mouse brainstem slices. Exp Brain Res 150, 117–121 (2003). https://doi.org/10.1007/s00221-003-1410-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1410-z