Abstract
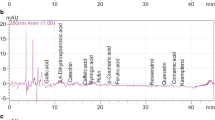
The present work aimed to evaluate three varieties of peanut (Arachis hypogaea L.) regarding the content of anthocyanins and total phenolics, antioxidant activity, phenolic profile and the alkaloids trigonelline and theobromine. The total phenolic content was analyzed by the Fast-Blue and Folin–Ciocalteau methods, while the antioxidant activity was analyzed by the β-carotene/linoleic acid, ABTS+ and FRAP methods. The phenolic profile and alkaloids were determined using high performance liquid chromatography (HPLC–DAD-UV–Vis). Peanuts with beige shells stood out with higher antioxidant activity and total phenolic content, while peanuts with purple shells stood out with higher anthocyanin content. Eight phenolic compounds and the alkaloids trigonelline and theobromine have been identified in peanut varieties. However, only four phenolics (gallic, syringic, ferulic and transcinnamic acids), in addition to the two alkaloids, were identified simultaneously in the three varieties studied. Chlorogenic acid was absent in red and purple-seeded varieties, catechin and resveratrol in varieties with red and beige coats, and p-coumaric acid in those with beige coats. For the first time, trigonelline and theobromine were identified in peanuts. Therefore, the bioactive composition and antioxidant activity of peanuts depend on its genotype.


Similar content being viewed by others
Data availability
Data will be available on request.
References
Settaluri VSK, Puppala CVK, Sundaram NJ (2012) Peanuts and their nutritional aspects—a review. Food Nutr Sci 3:1644–1650. https://doi.org/10.4236/fns.2012.312215
Guo C, Xie YJ, Zhu MT, Xiong Q, Chen Y, Yu Q, Xie JH (2020) Influence of different cooking methods on the nutritional and potentially harmful components of peanuts. Food Chem 316:1–9. https://doi.org/10.1016/j.foodchem.2020.126269
Statista (2023). Production share of peanuts worldwide in 2021, by leading country. https://www.statista.com/statistics/1030846/major-producers-of-peanut-worldwide/. Accessed 18 Feb 2024
Bodoira R, Rossi Y, Velez A, Montenegro M, Martínez M, Ribotta P, Maestri D (2022) Impact of storage conditions on the composition and antioxidant activity of peanut skin phenolic-based extract. Molecules 57(10):6471–6479. https://doi.org/10.1111/ijfs.15964
Companhia Nacional de Abastecimento (Conab) (2024). Amendoim. https://www.conab.gov.br/info-agro/safras/serie-historica-das-safras/itemlist/category/899-amendoim. Accessed 20 April 2024.
Çiftçi S, Suna G (2022) Functional components of peanuts (Arachis Hypogaea L.) and health benefits: a review. Future Foods 5:100140. https://doi.org/10.1016/j.fufo.2022.100140
Francisco MLDL, Resurreccion AVA (2008) Functional components in peanuts. Crit Rev Food Sci Nutr 48:715–746. https://doi.org/10.1080/10408390701640718
Jung M, Kim J, Ahn SM (2020) Factors associated with frequency of peanut consumption in Korea: a national population-based study. Nutrients 12(1207):1–15. https://doi.org/10.3390/nu12051207
Arya SS, Salve AR, Chauhan S (2016) Peanuts as functional food: a review. J Food Sci Technol 53(1):31–41. https://doi.org/10.1007/s13197-015-2007-9
Win M, Abdul Hamid A, Baharin B, Anwar F, Mc S, Dek M (2011) Phenolic compounds and antioxidant activity of peanut’s skin, hull, raw kernel and roasted kernel flour. Pak J Bot 43:1635–1642
Chen GH, Yang CY, Lee SJ, Wu CC, Tzen JTC (2014) Catechin content and the degree of its galloylation in oolong tea are inversely correlated with cultivation altitude. J Food Drug Anal 22:303–309. https://doi.org/10.1016/j.jfda.2013.12.001
Toomer OT (2018) Nutritional chemistry of the peanut (Arachis hypogaea). Crit Rev Food Sci Nutr 58(17):3042–3053. https://doi.org/10.1080/10408398.2017.1339015
Kyei SK, Akaranta O, Darko G (2020) Synthesis, characterization and antimicrobial activity of peanut skin extract-azo-compounds. Scientific African 8:1–14. https://doi.org/10.1016/j.sciaf.2020.e00406
Zhao X, Chen J, Du F (2012) Potential use of peanut by-products in food processing: a review. J Food Sci Technol 49(5):521–529. https://doi.org/10.1007/s13197-011-0449-2
Chuenchom PSP, Senawong T, Jogloy S (2016) Antioxidant capacity and phenolic content evaluation on peanut skins from 3 peanut types. Chiang Mai J Sci 43(1):123–137
Guo J, Chen H, Liu N, Chen W, Zhou X, Luo H, Huang L, Li W, Wu B, Huai D, Lei Y, Liao B, Jiang H (2022) Identification and validation of a major locus with linked marker for resveratrol content in culitivated peanut. Euphytica 218(15):1–10. https://doi.org/10.1007/s10681-022-02969-2
Nugrahini AD, Ishida M, Nakagawa T, Nishi K, Sugahara T (2020) Trigonelline: Na alkaloid with anti-degranulation properties. Mol Immunol 118:201–209. https://doi.org/10.1016/j.molimm.2019.12.020
Shojaei-Zarghani S, Yari Khosroushahi A, Rafraf M (2021) Oncopreventive effects of theanine and theobromine on dimethylhydrazine-induced colon cancer model. Biomed Pharmacother 134:1–9. https://doi.org/10.1016/j.biopha.2020.111140
Cadoná FC, Dantas RF, de Mello GH, Silva FP Jr (2022) Natural products targeting into cancer hallmarks: an update on caffeine, theobromine, and (+)–catechin. Food Sci Nutr 62(26):7222–7241. https://doi.org/10.1080/10408398.2021.1913091
Peerapen P, Boonmark W, Thongboonkerd V (2022) Trigonelline prevents kidney stone formation processes by inhibiting calcium oxalate crystallization, growth and crystal-cell adhesion, and downregulating crystal receptors. Biomed Pharmacother 149:1–14. https://doi.org/10.1016/j.biopha.2022.112876
Tanaka E, Mitani T, Nakashima M, Yonemoto E, Fujii H, Ashida H (2022) Theobromine enhances the conversion of white adipocytes into beige adipocytes in a PPARγ activation-dependent manner. J Nutr Biochem 100:1–11. https://doi.org/10.1016/j.jnutbio.2021.108898
Köppen W (1936) Das geographische system der klimatologie. Borntrager, Berlim, p 44
Climate-Data.Org. (2024) Clima Santana de Garambéu (Brasil). Disponível: https://pt.climate-data.org/america-do-sul/brasil/minas-gerais/santana-do-garambeu-176063/>. Accessed 22 Apr 2024.
Araújo AR, Oliveira JM, Pereira P, Curi N, Marques AFSM, Marques JJGSM (2019) Geomorfologia, solos e aptidão agrícola das terras da bacia do alto Rio Grande, Minas gerais. editora UFLA, Lavras, p 239
Barcia M, Pertuzatti P, Jacques A, Godoy H, Zambiazi R (2012) Bioactive compounds, antioxidant activity and percent composition of jambolão fruits (Syzygium cumini). Nat Prod J Shariah 2(2):129–138. https://doi.org/10.2174/2210315511202020129
Whaterhouse AL (2002) Polyphenlics: determination of total phenolic. In: Wrolstad RE (ed) Current protocols in food analytical chemistry. John Wiley, New York, pp 1–8
Paradiso VM, Castellino M, Renna M, Gattullo CE, Calasso M, Terzano R, Allegretta I, Leoni B, Caponio F, Santamaria P (2018) Nutritional characterization and shelf-life of packaged microgreens. Food Funct 9(11):5629–5640. https://doi.org/10.1039/C8FO01182F
Medina MB (2011) Determination of the total phenolics in juices and superfruits by a novel chemical method. J Funct Foods 3(2):79–87. https://doi.org/10.1016/j.jff.2011.02.007
Rufino MDSM, Alves RE, de Brito ES, Pérez-Jiménez J, Saura-Calixto F, Mancini-Filho J (2010) Bioactive compounds and antioxidante capacities of 18 no-traditional tropical fruits from Brazil. Food Chem 121(4):996–1002. https://doi.org/10.1016/j.foodchem.2010.01.037
Auzanneau N, Weber P, Kosińska-Cagnazzo A, Andlauer W (2018) Bioactive compounds and antioxidant capacity of Lonicera caerulea berries: comparison of seven cultivars over three harvesting years. J Food Compos Anal 66:81–89. https://doi.org/10.1016/j.jfca.2017.12.006
Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48(8):3396–3402. https://doi.org/10.1021/jf9913458
Ferreira DF (2010) SISVAR–Sistema de análise de variância. In (Version 5.8) https://des.ufla.br/~danielff/programas/sisvar.html. Accessed 1 Dec 2023
Nunes CA, Freitas MP, Pinheiro ACM, Bastos SC (2012) Chemoface: a novel free user-friendly interface for chemometrics. J Braz Chem Soc 23(11):2003–2010. https://doi.org/10.1590/S0103-50532012005000073
Attree R, Du B, Xu B (2015) Distribution of phenolic compounds in seed coat and cotyledon, and their contribution to antioxidant capacities of red and black seed coat peanuts (Arachis hypogaea L.). Ind Crops Prod 67:448–456. https://doi.org/10.1016/j.indcrop.2015.01.080
Kuang Q, Yu Y, Attree R, Xu B (2017) A comparative study on anthocyanin, saponin, and oil profiles of black and red seed coat peanut (Arachis hypogacea) grown in China. Int J Food Prop 20(1):131–140. https://doi.org/10.1080/10942912.2017.1291676
Yang QQ, Kim GAKF, Luo Q, Corke H (2020) Phenolic profile, antioxidant and antiproliferative activities of diverse peanut cultivars. J Food Measure Characteriz 14:2361–2369. https://doi.org/10.1007/s11694-020-00483-4
Camargo AC, Regitano-d’Arce MAB, Shahidi F (2017) Phenolic profile of peanut by-products: antioxidant potential and inhibition of alfa-glucosidase and lipases activities. J Am Oil Chem Soc 94:959–971. https://doi.org/10.1007/s11746-017-2996-9
Zhou Z, Fan Z, Meenu M, Xu B (2021) Impact of germination time on resveratrol, phenolic acids, and antioxidant capacities of different varieties of peanut (Arachis hypogaea Linn.) from China. Antioxidants 10:1714
Fernandes FHA, Salgado HRN (2016) Gallic acid: review of the methods of determination and quantification. Anal Chem 46:257–267. https://doi.org/10.1080/10408347.2015.1095064
Kahkeshani N, Farzaei F, Fotouhi M, Alavi SS, Bahramsoltani R, Naseri R, Momtaz S, Abbasabadi Z, Rahimi R, Farzaei MH, Bishayee A (2019) Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci 22(3):225–237. https://doi.org/10.22038/ijbms.2019.32806.7897
Wang L, Pan X, Jiang L, Chu Y, Gao S, Jiang X, Zhang Y, Chen Y, Luo S, Peng C (2022) The biological activity mechanism of chlorogenic acid and its applications in food industry: a review. Front Nutr 9(943911):1–22. https://doi.org/10.3389/fnut.2022.943911
Srinivasulu C, Ramgopal M, Ramanjaneyulu G, Anuradha CM, Suresh Kumar C (2018) Syringic acid (SA)–a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed Pharmacother 108:547–557. https://doi.org/10.1016/j.biopha.2018.09.069
Li D, Rui YX, Guo SD, Luan F, Liu R, Zeng N (2021) Ferulic acid: a review of its pharmacology, pharmacokinetics and derivatives. Life Sci 284:1–13. https://doi.org/10.1016/j.lfs.2021.119921
Zhang L, Qu H, Xie M, Shi T, Shi P, Yu M (2023) Effects of different cooking methods on phenol content and antioxidant activity in sprouted peanut. Molecules 28(12):4684
Wang Z, Ge S, Li S, Lin H, Lin S (2020) Anti-obesity effect of trans-cinnamic acid on HepG2 cells and HFD-fed mice. Food Chem Toxicol 137:111148. https://doi.org/10.1016/j.fct.2020.111148
Adisakwattana S (2017) Cinnamic acid and its derivatives: mechanisms for prevention and management of diabetes and its complications. Nutrients 9(163):1–27
Hseu YC, Korivi M, Lin FY, Li ML, Lin RW, Wu JJ, Yang HL (2018) Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of Nrf2-mediated antioxidant genes in human skin fibroblastos. J Dermatol Sci 90:123–134. https://doi.org/10.1016/j.jdermsci.2018.01.004
Putra NR, Rizkiyah DN, Qomariyah L, Aziz AHA, Veza I, Yunus MAC (2023) Experimental and modeling for catechin and epicatechinrecovery from peanut skin using subcritical etanol. J Food Process Eng 46(e14275):1–11. https://doi.org/10.1111/jfpe.14275
Pedro A, Maciel GM, Ribeiro V, Haminiuk C (2019) Fundamental and applied aspects of catechins from differentsources: a reviews. Int J Food Sci Technol 55(2):429–442. https://doi.org/10.1111/ijfs.14371
Phan-Thien KY, Wright GC, Lee NA (2014) Peanut antioxidants: Part 2. Quantitation of free and matrix-bound phytochemicals in five selected genotypes with diverse antioxidante capacity by high performance liquid chromatog-raphy (HPLC). LWT Food Sci Technol 57:312–319. https://doi.org/10.1016/j.lwt.2013.12.020
Parvizi F, Yaghmaei P, Haeri Rohani SA, Mard AS (2020) Hepatoprotective properties of p-coumaric acid in a rat model of ischemia-reperfusio. Avicenna J Phytomed 10(6):633–640
Boo YC (2019) p-coumaric acid as an active ingredient in cosmetics: a review focusing on its antimelanogenic effects. Antioxidants 8(275):1–16
Rafiee Z, Moaiedi MZ, Gorji AV, Mansouri E (2020) Mitigates doxorubicin-induced nephrotoxicity through suppression of oxidative stress, inflammation and apoptosis. Arch Med Res 51:32–40. https://doi.org/10.1016/j.arcmed.2019.12.004
Rudolf JR, Resurreccion AVA (2005) Elicitation of resveratrol in peanut kernels by application of abiotic stresses. J Agric Food Chem 53:10186–10192. https://doi.org/10.1021/jf0506737
Yin Y, Hu J, Yang Z, Fang W, Yang J (2023) Effects of methyl jasmonate and NaCl treatments on the resveratrol accumulation and defensive responses in germinated peanut (Arachis hypogaea L.). Plant Physiol Biochem 194:664–673. https://doi.org/10.1016/j.plaphy.2022.12.012
Sanders TH, McMichael RW, Hendrix KW (2000) Occurrence of resveratrol in edible peanuts. J Agric Food Chem 48:1243–1246. https://doi.org/10.1021/jf990737b
Peláez PP, Bardón I, Camasca P (2016) Methylxanthine and catechin content of fresh and fermented cocoa beans, dried cocoa beans, and cocoa liquor. Sci Agropec 7(4):355–365. https://doi.org/10.17268/sci.agropecu.2016.04.01
Konstantinidis N, Franke H, Schwarz S, Lachenmeier DW (2023) Risk assessment of trigonelline in coffee and coffee by-products. Molecules 28(3460):1–22. https://doi.org/10.3390/molecules28083460
Wootton-Beard PC, Moran A, Ryan L (2011) Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Int 44(1):217–224. https://doi.org/10.1016/j.foodres.2010.10.033
Rumpf J, Burger R, Schulze M (2023) Statistical evaluation of DPPH, ABTS, FRAP, and folin-ciocalteu assays to assess the antioxidant capacity of lignins. Int J Biol Macromol 233:1–9. https://doi.org/10.1016/j.ijbiomac.2023.123470
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins Byrne D (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19(6):669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Marinova EM, Toneva A, Yanishlieva N (2009) Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem 114:1498–1502. https://doi.org/10.1016/j.foodchem.2008.11.045
Tounekti T, Joubert E, Hernández I, Munné-Bosch S (2013) Improving the polyphenol content of tea. Crit Rev Plant Sci 32(3):192–215. https://doi.org/10.1080/07352689.2012.747384
Tanaka T, Matsuo Y, Kouno I (2020) Chemistry of secondary polyphenols produced during processing of tea and selected foods. Int J Mol Sci 11(1):14–40
Rauf A, Imran M, Abu-Izneid T, Haq IU, Patel S, Pan X, Naz S, Silva AS, Saeed F, Suleria HAR (2019) Proanthocyanidins: a comprehensive review. Biomed Pharmacother 116:1–6. https://doi.org/10.1016/j.biopha.2019.108999
Liu C, Wang X, Shulaev V, Dixon RA (2016) A role for leucoanthocyanidin reductase in the extension of proanthocyanidins. Nature plants 2:1–7. https://doi.org/10.1038/nplants.2016.182
Uysal RS, Issa-Issa H, Sendra E, Carbonell-Barrachina ÁA (2023) Changes in anthocyanin pigments, trans-resveratrol, and colorimetric characteristics of Fondillón wine and other “Monastrell” wines during the aging period. Eur Food Res Technol 249(7):1821–1831. https://doi.org/10.1007/s00217-023-04256-3
Acknowledgements
The authors would like to thank the Central of Analysis and Chemical Prospecting of the Federal University of Lavras, and Finep, To The National Council of Technological and Scientific Development (CNPq:304413/2016-0; 302699/2019-8), the Minas Gerais Research Support Foundation (FAPEMIG: PPM-00458-15; PPM-00355-17), and the Higher Education Personnel Improvement Coordination (CAPES: 88881.068456/2014-01) for financial support.
Author information
Authors and Affiliations
Contributions
Gilson Gustavo Lucinda Machado: Conceptualization, Investigation, Methodology, Data curation, Formal analysis, Writing – original draft. Ana Beatriz Silva Araújo: Conceptualization, Formal Analysis. Ana Cristina Freitas de Oliveira Meira: Conceptualization, Formal analysis. Carlos Henrique Milagres Ribeiro: Conceptualization, Formal analysis. Ingrid Alves Santos: Conceptualization, Formal analysis. Lorrane Ribeiro de Souza: Conceptualization, Formal analysis. Elano Pinheiro Pereira: Conceptualization, Formal analysis. Eduardo Valério de Barros Vilas Boas: Conceptualization, Fundraising, Project Administration, Supervision, Validation, Visualization, Writing—Original Draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical requirements
This article does not contain any studies with human or animal subject.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Machado, G.G.L., Araújo, A.B.S., de Oliveira Meira, A.C.F. et al. Bioactive capacity of peanuts with different coat colors. Eur Food Res Technol (2024). https://doi.org/10.1007/s00217-024-04572-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00217-024-04572-2