Abstract
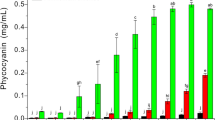
Different methods were used to extract C-phycocyanin from Arthrospira platensis, alone or in synergy (freezing, incubation, ultrasounds, deep eutectic solvents). Freezing or Freeze-drying spirulina allows a highly facilitated rupture of the cell membranes. Incubation in water allows then a quick release of C-PC and other phycobiliproteins at 45 °C (2 h) while it takes less than 8 h at 35 °C and 25–30 h at ambient temperature. In addition to microscopic observation, the evolution of solutes concentration with incubation time confirms that hydrosoluble pigments are first preferentially released relatively to chlorophyll which requires deeper collapse of the spirulina cells. Use of ultrasounds allows an extraction of the spirulina content in 15 min of treatment with a high C-PC extraction yield (> 200 mg/g) and good protein purity (around 0.7). Ethyl acetate, ethanol and a mixture of these solvents give good extraction yields of chlorophyll (around 21 mg/g). C-PC extraction yield is yet then decreased due to a denaturation of the hydrated pigment. Fructose can be used to prevent denaturation by both organic solvent and freezing/freeze-drying. The use of deep-eutectic solvents is not recommended here as no better yield or selectivity are achieved compared to extraction in buffered water.















Similar content being viewed by others
References
Fernández-Rojas B, Hernández-Juárez J, Pedraza-Chaverri J (2014) Nutraceutical properties of phycocyanin. J Funct Foods 11:375–392. https://doi.org/10.1016/j.jff.2014.10.011
Eriksen NT (2008) Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14. https://doi.org/10.1007/s00253-008-1542-y
Liu Q, Huang Y, Zhang R et al (2016) Medical Application of Spirulina platensis derived C-phycocyanin. Eviden Based Complem Alternat Med 2016:1–14. https://doi.org/10.1155/2016/7803846
Abd El-Baky HH (2003) Over production of phycocyanin pigment in blue green alga Spirulina sp. and lt’s inhibitory effect on. J Med Sci 3(4):314–24. https://doi.org/10.3923/jms.2003.314.324
Safi C, Ursu AV, Laroche C et al (2014) Aqueous extraction of proteins from microalgae: effect of different cell disruption methods. Algal Res 3:61–65. https://doi.org/10.1016/j.algal.2013.12.004
Chaiklahan R, Chirasuwan N, Loha V et al (2011) Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Biores Technol 102:7159–7164. https://doi.org/10.1016/j.biortech.2011.04.067
Pan-utai W, Kahapana W, Iamtham S (2018) Extraction of C-phycocyanin from Arthrospira (Spirulina) and its thermal stability with citric acid. J Appl Phycol 30:231–242. https://doi.org/10.1007/s10811-017-1155-x
Silveira ST, Burkert JFM, Costa JAV et al (2007) Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Biores Technol 98:1629–1634. https://doi.org/10.1016/j.biortech.2006.05.050
Sala L, Kalil SJ, Moraes CC (2018) Cell pretreatment with ethylenediaminetetraacetic acid for selective extraction of C-phycocyanin with food grade purity. Biotechnol Prog 34:1261–1268. https://doi.org/10.1002/btpr.2713
Sharma R, Bhunia B, Mondal A et al (2020) Statistical optimization of process parameters for improvement of phycobiliproteins (PBPs) yield using ultrasound-assisted extraction and its kinetic study. Ultrason Sonochem 60:104762
Tavanandi HA, Mittal R, Chandrasekhar J, Raghavarao KSMS (2018) Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res 31:239–251. https://doi.org/10.1016/j.algal.2018.02.008
Fabre J-F, Niangoran NUF, Gaignard C et al (2022) Extraction, purification and stability of C-phycocyanin from Arthrospira platensis. Eur Food Res Technol 248:1583–1599. https://doi.org/10.1007/s00217-022-03987-z
Marzorati S, Schievano A, Idà A, Verotta L (2020) Carotenoids, chlorophylls and phycocyanin from Spirulina: supercritical CO2 and water extraction methods for added value products cascade. Green Chem 22:187–196. https://doi.org/10.1039/C9GC03292D
Minchev I, Petkova N, Milkova-Tomova I (2020) Ultrasound-assisted extraction of chlorophylls and phycocyanin from Spirulina platensis. Biointerface Res Appl Chem 11:9296–9304. https://doi.org/10.33263/BRIAC112.92969304
Vendruscolo RG, Fernandes AS, Fagundes MB et al (2021) Development of a new method for simultaneous extraction of chlorophylls and carotenoids from microalgal biomass. J Appl Phycol 33:1987–1997. https://doi.org/10.1007/s10811-021-02470-8
Manirafasha E, Murwanashyaka T, Ndikubwimana T et al (2017) Ammonium chloride: a novel effective and inexpensive salt solution for phycocyanin extraction from Arthrospira (Spirulina) platensis. J Appl Phycol 29:1261–1270. https://doi.org/10.1007/s10811-016-0989-y
Zhang X, Zhang F, Luo G et al (2014) Extraction and separation of phycocyanin from Spirulina using aqueous two-phase systems of ionic liquid and salt. JFNR 3:15–19. https://doi.org/10.12691/jfnr-3-1-3
Zhao L, Peng Y, Gao J, Cai W (2014) Bioprocess intensification: an aqueous two-phase process for the purification of C-phycocyanin from dry Spirulina platensis. Eur Food Res Technol 238:451–457. https://doi.org/10.1007/s00217-013-2124-5
Boussiba S, Richmond AE (1979) Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch Microbiol 120:155–159. https://doi.org/10.1007/BF00409102
Lauceri R, Bresciani M, Lami A, Morabito G (2017) Chlorophyll a interference in phycocyanin and allophycocyanin spectrophotometric quantification. J Limnol. https://doi.org/10.4081/jlimnol.2017.1691
Rowan KS (1989) Photosynthetic pigments of algae. University Press, Cambridge
Wintermans JFGM, De Mots A (1965) Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochimica et Biophysica Acta (BBA) Biophys Including Photosynth 109:448–453. https://doi.org/10.1016/0926-6585(65)90170-6
Wellburn AR (1994) The spectral determination of Chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Seely GR, Jensen RG (1965) Effect of solvent on the spectrum of chlorophyll. Spectrochim Acta 21:1835–1845. https://doi.org/10.1016/0371-1951(65)80095-9
Zarrouk C (1966) Contribution a l’etude d’une Cyanophycee. In : Influence de Divers Facteurs Physiques et Chimiques sur la croissance et la photosynthese de Spirulina mixima. Thesis University of Paris, France
Fabre J-F, Lacroux E, Valentin R, Mouloungui Z (2015) Ultrasonication as a highly efficient method of flaxseed mucilage extraction. Ind Crops Prod 65:354–360. https://doi.org/10.1016/j.indcrop.2014.11.015
Peleg M (1988) An empirical model for the description of moisture sorption curves. J Food Sci 53:1216–1217. https://doi.org/10.1111/j.1365-2621.1988.tb13565.x
Xu K, Xu P, Wang Y (2020) Aqueous biphasic systems formed by hydrophilic and hydrophobic deep eutectic solvents for the partitioning of dyes. Talanta 213:120839. https://doi.org/10.1016/j.talanta.2020.120839
Liu Y, Li J, Fu R et al (2019) Enhanced extraction of natural pigments from Curcuma longa L. using natural deep eutectic solvents. Ind Crops Prod 140:111620. https://doi.org/10.1016/j.indcrop.2019.111620
Zuo J, Geng S, Kong Y et al (2023) Current progress in natural deep eutectic solvents for the extraction of active components from plants. Crit Rev Anal Chem 53:177–198. https://doi.org/10.1080/10408347.2021.1946659
Hilali S, Wils L, Chevalley A et al (2022) Glycerol-based NaDES as green solvents for ultrasound-assisted extraction of phycocyanin from Arthrospira platensis—RSM optimization and ANN modelling. Biomass Conv Bioref 12:157–170. https://doi.org/10.1007/s13399-021-02263-6
Pez Jaeschke D, Rocha Teixeira I, Damasceno Ferreira Marczak L, Domeneghini Mercali G (2021) Phycocyanin from Spirulina: a review of extraction methods and stability. Food Res Int 143:110314. https://doi.org/10.1016/j.foodres.2021.110314
Funding
The funding has been received from FEDER with Grant no. ESR_R&S_DI-000173.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Compliance with ethics requirements
This study does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fabre, JF., Niangoran, N.U.F., Gaignard, C. et al. Extraction of C-PC from Arthrospira platensis: use of ultrasounds, organic solvents and deep eutectic solvents. Eur Food Res Technol 250, 1149–1161 (2024). https://doi.org/10.1007/s00217-023-04452-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04452-1