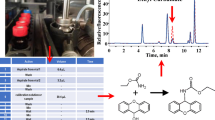
Abstract
The detection and analysis of α-dicarbonyl compounds (α-DCs) in food can guide the daily diet of patients with various chronic diseases. However, these compounds have poor stability, lack color-generating groups, and have low ionization efficiency. Therefore, it is difficult to monitor them. We established a new simple and sensitive method for the simultaneous determination of four α-DCs, including glyoxal (GO), methylglyoxal (MG), 2,3-butanedione (DMGO), and 3-deoxyglucosone (3-DG) in beer by ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) with pre-column derivatization using 3-benzyl-2-oxo-4λ3-thiazolidine-4-carbohydrazide (BOTC), a novel MS probe for targeted identification of carbonyl compounds. α-DCs were derivatized with BOTC at 60 °C for 60 min, and the gradient analysis was carried out on a CSH C18 (2.1 mm × 50 mm; 1.7 μm) column for 5 min with a mobile phase A of 0.1% formic acid (FA) in water and mobile phase B of 0.1% FA in acetonitrile. The linearity range of this method was 0.39–500 μM (R2 ≥ 0.9995). The limits of detection (LOD) were in the range of 13–50 fmol. The average recoveries of α-DCs were 95.15–101.67%. This method was successfully applied to the detection of α-DCs in beer; the content of 3-DG was the highest, several hundred times higher than that of GO, MG, and DMGO, and the difference was significant. This new BOTC derivatization-based UHPLC-MS/MS method for the simultaneous detection of four α-DCs in beer is highly sensitive and can be used for the detection of α-DCs in alcoholic beverages.
Graphical abstract





Similar content being viewed by others
References
Olas B, Bryś M (2020) Beer components and their beneficial effect on the hemostasis and cardiovascular diseases-truth or falsehood. Food Chem Toxicol 146:111782. https://doi.org/10.1016/j.fct.2020.111782
Eldarov MA, Kishkovskaia SA, Tanaschuk TN, Mardanov AV (2016) Genomics and biochemistry of saccharomyces cerevisiae wine yeast strains. Biochemistry (Mosc) 81:1650–1668. https://doi.org/10.1134/S0006297916130046
Cha J, Debnath T, Lee KG (2019) Analysis of α-DCs and volatiles formed in Maillard reaction model systems. Sci Rep 9:5325. https://doi.org/10.1038/s41598-019-41824-8
Brings S, Fleming T, Freichel M, Muckenthaler MU, Herzig S, Nawroth PP (2017) Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int J Mol Sci 18:984. https://doi.org/10.3390/ijms18050984
Schalkwijk CG (2015) Vascular AGE-ing by methylglyoxal: the past, the present and the future. Diabetologia 58:1715–1719. https://doi.org/10.1007/s00125-015-3597-5
Zadeh RG, Yaylayan V (2020) Exploring the scope of indole interaction with 1,2-dicarbonyl compounds. Food Chem 327:127031. https://doi.org/10.1016/j.foodchem.2020.127031
Bravo A, Herrera JC, Scherer E, Ju-Nam Y, Rübsam H, Madrid J, Zufall C, Rangel-Aldao R (2008) Formation of alpha-dicarbonyl compounds in beer during storage of pilsner. J Agric Food Chem 56:4134–4144. https://doi.org/10.1021/jf703696p
Maasen K, Scheijen JLJM, Opperhuizen A, Stehouwer CDA, Van Greevenbroek MM, Schalkwijk CG (2021) Quantification of dicarbonyl compounds in commonly consumed foods and drinks; presentation of a food composition database for dicarbonyls. Food Chem 339:128063. https://doi.org/10.1016/j.foodchem.2020.128063
Wang XJ, Ma SB, Liu ZF, Li H, Gao WY (2019) Elevated levels of α-DCs in the plasma of type II diabetics and their relevance with diabetic nephropathy. J Chromatogr B 1106–1107:19–25. https://doi.org/10.1016/j.jchromb.2018.12.027
Rowan S, Bejarano E (1864) Taylor A (2018) Mechanistic targeting of advanced glycation end-products in age-related diseases. Biochim Biophys Acta Mol Basis Dis 12:3631–3643. https://doi.org/10.1016/j.bbadis.2018.08.036
Henning C, Liehr K, Girndt M, Ulrich C, Glomb MA (2014) Extending the spectrum of α-DCs in vivo. J Biol Chem 289:28676–28688. https://doi.org/10.1074/jbc.m114.563593
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9. https://doi.org/10.2337/diabetes.48.1.1
Chatterjee S, Chen A (2012) Voltammetric detection of the α-dicarbonyl compound: Methylglyoxal as a flavoring agent in wine and beer. Anal Chim Acta 751:66–70. https://doi.org/10.1016/j.aca.2012.09.011
Do Rosário PM, Cordeiro CA, Freire AP, Nogueira JM (2005) Analysis of methylglyoxal in water and biological matrices by capillary zone electrophoresis with diode array detection. Electrophoresis 26:1760–1767. https://doi.org/10.1002/elps.200410216
Li P, Zhu Y, He S, Fan J, Hu Q, Cao Y (2012) Development and validation of a high-performance liquid chromatography method for the determination of diacetyl in beer using 4-nitro-o-phenylenediamine as the derivatization reagent. J Agric Food Chem 60:3013–3019. https://doi.org/10.1021/jf3007163
Jeong JH, Cha J, Lee KG (2017) Validation of analytical method for α-dicarbonyl compounds using gas chromatography–nitrogen phosphorous detector and their levels in alcoholic beverages. Int J Food Sci Tech 52:1491–1497. https://doi.org/10.1111/ijfs.13414
Ma Q, Qi C, Li X-L, Shi Q, Xu C-Y, Jin T, Min JZ (2021) Simultaneous determination of DL-cysteine, DL-homocysteine, and glutathione in saliva and urine by UHPLC-Q-Orbitrap HRMS: application to studies of oxidative stress. J Pharmaceut Biomed 196:113939. https://doi.org/10.1016/j.jpba.2021.113939
Zhang J, Wei F, Zhang T, Peng B, Zhang Y, Wang S, Cui M (2022) Simultaneous determination of seven α-DCs in milk and milk products based on an LC–MS/MS method with matrix-matched calibration. Food Anal Method 15:1652–1662. https://doi.org/10.1007/s12161-021-02219-6
Zhang TY, Li S, Zhu QF, Wang Q, Hussain D, Feng YQ (2019) Derivatization for liquid chromatography-electrospray ionization-mass spectrometry analysis of small-molecular weight compounds. Trac-Trend Anal Chem 119:115608. https://doi.org/10.1016/j.trac.2019.07.019
Rodríguez-Cáceres MI, Palomino-Vasco M, Mora-Diez N, Acedo-Valenzuela MI (2017) Dispersive liquid-liquid microextraction for a rapid determination of glyoxal in alcoholic beverages. Talanta 168:100–104. https://doi.org/10.1016/j.talanta.2017.03.031
Rodríguez-Cáceres MI, Palomino-Vasco M, Mora-Diez N, Acedo-Valenzuela MI (2015) Novel HPLC-Fluorescence methodology for the determination of methylglyoxal and glyoxal. Application to the analysis of monovarietal wines “Ribera del Guadiana.” Food Chem 187:159–165. https://doi.org/10.1016/j.foodchem.2015.04.103
El-Maghrabey MH, Nakatani T, Kishikawa N, Kuroda N (2018) Aromatic aldehydes as selective fluorogenic derivatizing agents for α-dicarbonyl compounds. application to HPLC analysis of some advanced glycation end products and oxidative stress biomarkers in human serum. J Pharmaceut Biomed 158:38–46. https://doi.org/10.1016/j.jpba.2018.05.012
Lim HH, Shin HS (2020) In-solution derivatization and detection of glyoxal and methylglyoxal in alcoholic beverages and fermented foods by headspace solid-phase microextraction and gas chromatography-mass spectrometry. J Food Compos Anal 92:103584. https://doi.org/10.1016/j.jfca.2020.103584
Hurtado-Sánchez MDC, Espinosa-Mansilla A, Rodríguez-Cáceres MI, Durán-Merás I (2014) Evaluation of liquid chromatographic behavior of lumazinic derivatives, from α-DCs, in different C18 columns: application to wine samples using a fused-core column and fluorescence detection. J Agric Food Chem 62:97–106. https://doi.org/10.1021/jf404180t
Cengiz S, Kişmiroğlu C, Çebi N, Çatak J, Yaman M (2020) Determination of the most potent precursors of advanced glycation end products (AGEs) in chips, crackers, and breakfast cereals by high performance liquid chromatography (HPLC) using precolumn derivatization with 4-nitro-1,2-phenlenediamine. Microchem J 2:2. https://doi.org/10.1016/j.microc.2020.105170
Hurtado-Sánchez MDC, Espinosa-Mansilla A, Durán-Merás I (2015) Influence of the presence of natural monosaccharides in the quantification of α-DCs in high content sugar samples. a comparative study by ultra-high performance liquid chromatography-single quadrupole mass spectrometry using different derivatization reactions. J Chromatogr A 1422:117–127. https://doi.org/10.1016/j.chroma.2015.10.001
Wang JY, Wang XJ, Hui X, Hua SH, Li H, Gao WY (2017) Determination of diacetyl in beer by a precolumn derivatization-HPLC-UV method using 4-(2,3-dimethyl-6-quinoxalinyl)-1,2-benzenediamine as a derivatizing reagent. J Agric Food Chem 65(12):2635–2641. https://doi.org/10.1021/acs.jafc.7b00990
Gobert J, Glomb MA (2009) Degradation of glucose: reinvestigation of reactive α-dicarbonyl compounds. J Agric Food Chem 57:8591–8597. https://doi.org/10.1021/jf9019085
Jiang Y, Hengel M, Pan C, Seiber JN, Shibamoto T (2013) Determination of toxic α-DCs, glyoxal, methylglyoxal, and diacetyl, released to the headspace of lipid commodities upon heat treatment. J Agric Food Chem 61:1067–1071. https://doi.org/10.1021/jf3047303
Min JZ, Yamamoto M, Yu H, Higashi T, Toyo’oka T, (2012) Rapid and sensitive determination of the intermediates of advanced glycation end products in the human nail by ultra-performance liquid chromatography with electrospray ionization time-of-flight mass spectrometry. Anal Biochem 424:187–194. https://doi.org/10.1016/j.ab.2012.02.025
Daglia M, Papetti A, Aceti C, Sordelli B, Spini V, Gazzani G (2007) Isolation and determination of α-DCs by RP HPLC-DAD in green and roasted coffee. J Agric Food Chem 55:8877–8882. https://doi.org/10.1021/jf071917l
Ojeda AG, Wrobel K, Escobosa AR, Garay-Sevilla ME, Wrobel K (2014) High-performance liquid chromatography determination of glyoxal, methylglyoxal, and diacetyl in urine using 4-methoxy-o-phenylenediamine as derivatizing reagent. Anal Biochem 449:52–58. https://doi.org/10.1016/j.ab.2013.12.014
Wang XJ, Zhang HX, Li H, Zhu AH, Gao WY (2019) Measurement of α-DCs in human saliva by pre-column derivatization HPLC. Clin Chem Lab Med 57:1915–1922. https://doi.org/10.1515/cclm-2019-0350
Wang XJ, Wang JY, Li H, Hui X, Gao WY (2017) Determination of diacetyl in liquors by high performance liquid chromatography coupled with precolumn derivatization using 3,3 ’-diaminobenzidine. Chin J Chromatogr 35(8):837–842. https://doi.org/10.3724/SP.J.1123.2017.04031
Weigel KU, Opitz T, Henle T (2004) Studies on the occurrence and formation of 1,2-dicarbonyls in honey. Eur Food Res Technol 218(2):147–151. https://doi.org/10.1007/s00217-003-0814-0
Akira K, Matsumoto Y, Hashimoto T (2004) Determination of urinary glyoxal and methylglyoxal by high-performance liquid chromatography. Clin Chem Lab Med 42(2):147–153. https://doi.org/10.3746/pnf.2014.19.2.098
Wang M, Liu Y, Guo B, Zhang F, Chou F, Ma M, Huang L, Luo Z, Chen B, Chen X (2021) Isotope-coding derivatization for quantitative profiling of reactive α-dicarbonyl species in processed botanicals by liquid chromatography–tandem mass spectrometry. J Agric Food Chem 69(35):10379–10393. https://doi.org/10.1021/acs.jafc.1c04122
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (32160234), the Science and Technology Development Project of Jilin Province of China (YDZJ202201ZYTS596). We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, ZG., Zhang, L., Qi, C. et al. A novel reagent pre-column derivatization method for UHPLC-MS/MS to determine four α-dicarbonyl compounds in beer. Eur Food Res Technol 249, 2017–2027 (2023). https://doi.org/10.1007/s00217-023-04269-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04269-y