Abstract
To assess the impact of black-colored grain on Alternaria mycotoxin concentrations in different stages of the brewing process, brewing experiments were conducted in a microscale brewhouse. Different mixtures of visually unaffected and black-colored batches of two malt samples were used, which were obtained by an optical sorting device. The 13 Alternaria mycotoxins alternariol (AOH), alternariol monomethyl ether (AME), tenuazonic acid (TeA), tentoxin (TEN), alterperylenol (ALTP), altertoxins I and II (ATX I and II), altenuene (ALT) as well as the modified forms AOH-3-glucoside (AOH-3-G), AOH-9-glucoside (AOH-9-G), AME-3-gluoside (AME-3-G), AOH-3-sulfate (AOH-3-S) and AME-3-sulfate (AME-3-S) were analyzed in each processing step by liquid chromatography–tandem mass spectrometry (LC–MS/MS), and the toxin concentrations were balanced over the whole brewing process. Fungal DNA content in the starting material (mixtures) was determined by quantitative real-time polymerase chain reaction (qPCR). In this study, TeA was the only toxin to migrate into the final beer, while the AOH, AME, TEN, ALTP and ATX I toxins were mainly found in the spent grains. The observance of AOH-3-S and AME-3-S in some processing steps also showed the possibility of modification reactions during brewing. Furthermore, no distinct correlations between the fungal DNA and the analyzed mycotoxins could be observed in the starting material, while the amount of black colored grains only impacted toxin concentrations in one of the two used malt samples. Nevertheless, it was shown that optical sorting of malt batches might be a useful tool for the malting and brewing industry to prevent elevated mycotoxin concentrations.
Similar content being viewed by others
Introduction
In Germany, beer is produced according to the “German Beer Purity Law” established in 1516, which stipulates that beer must contain nothing other than water, malt, hops and, later, yeast. The primary ingredient is malt, as it provides starch that is hydrolyzed into fermentable sugars, which are essential for subsequent fermentation. Worldwide, 90% of malt is made from barley [1]. The two-rowed barley is the most common form for malting, since the grains are developed symmetrically and are generally larger and rounder than the six-rowed barley variety [2, 3]. The most important quality criteria that make a barley sample perfectly suited for brewing are an optimal starch-to-protein-ratio, high enzymatic potential, and a high germination capacity [4]. However, the batch must also be flawless from a microbiological point of view. As a natural product, barley is susceptible to fungal infestation, which may lead to yield losses and reduced processing quality.
Apart from the technological problems related to handling of contaminated cereals, processing is also connected with legal risks for brewers as well as potential health risks for consumers [5]. Various fungal species are capable of producing a wide range of toxic secondary metabolites (mycotoxins), which could be transferred from the grains into the final beer during brewing. Previous studies that focused on the widely distributed fungal genus Fusarium showed a transfer of Fusarium toxins into the beer and its by-products, depending on their solubility [4, 6,7,8,9]. Besides Fusarium species, other mycotoxigenic fungi are known to colonize malting barley. As previously described, fungi of the genus Alternaria constitute a large part of the barley mycoflora [10]. Accordingly, in a recent study, various Alternaria toxins have been detected in brewing malts [11].
The genus Alternaria includes ubiquitous filamentous fungi with a saprophytic or (opportunistic) plant pathogenic lifestyle, commonly found in soil or on decaying plant tissues. About 300 species are known to infest a large variety of agricultural crops, as well as causing postharvest spoilage [12,13,14]. The most important crop damaging representatives include: Alternaria alternata (syn. A. tenuis), A. tenuissima, A. solani, A. citri, A. infectoria, A. arborescens, A. brassicae and A. brassicola, from which mainly A. alternata is known for causing black point disease on cereal kernels and is, therefore, responsible for the black discoloration of the raw material [15, 16]. Within the genus, A. alternata was identified as the most important toxin producer; it is the only known species that can produce all targeted toxins including alternariol (AOH), alternariol monomethyl ether (AME) and alterperylenol (ALT), as well as tenuazonic acid (TeA), tentoxin (TEN) and the altertoxins I and II (ATX I and II) [5]. So far, no legal regulations have been currently established for Alternaria mycotoxins due to insufficient data, showing the need for more research in this area.
Until now, TeA, AOH and AME were the only Alternaria toxins to be found in the final beer, which indicates a mycotoxin transfer of these three toxins during brewing [17,18,19,20]. However, a recent study on mycotoxin concentrations in 50 representative malt samples could show the presence of other relevant Alternaria toxins such as ALTP, ATX I and, to some extent, also the modified form AOH-3-sulfate (AOH-3-S) in this raw material [11]. This finding emphasizes the need to pursue the concentrations of these toxins during the brewing process as well as to evaluate why none of these toxins have been detected in beer so far. Furthermore, the fate of the toxins AOH, AME, TEN and TeA were recently investigated during brewing already, and results emphasized the need to track other Alternaria mycotoxins as well [6].
Since cereal quality and safety are of major importance to the processing industry, several strategies have been proposed to reduce fungal and mycotoxin contamination. One promising approach is optical sorting technology. Fungal infestation leads to several changes on cereal kernels, such as structural and color changes, which can be of use to detect and remove discolored kernels from the batch by optical sorting technology with the intention to decrease the mycotoxin content [21]. Sorting maize kernels for aflatoxin reduction has been proven [22], but to date, there are no data on Alternaria spp. contamination and associated mycotoxins in black-colored malt kernels available.
In this study, brewing trials with defined dosages of contaminated black kernels were carried out to monitor the fate of Alternaria mycotoxins throughout the brewing process depending on the initial raw material contamination. For this purpose, naturally infected barley malt batches were separated by an optical sorting device into a visually clean, “healthy” fraction, which was used as a reference, and a black-colored symptomatic fraction. By remixing, batches of varying proportions were generated, on the basis of which the effect of purification was investigated in terms of the relationships between black symptomatic kernels, total fungal DNA and levels of mycotoxins. To determine the amount of A. alternata DNA, a previously established qPCR assay based on SYBR green technology for the detection and quantification of black fungal species on malting barley raw material was used [10]. To follow up on the concentration of Alternaria toxins throughout the brewing process, we applied the recently developed multi-mycotoxin liquid chromatography tandem mass spectrometry (LC–MS/MS) methods for cereals and beer [11, 20] for the analysis of TeA, AOH, AME, TEN, altenuene (ALT), ALTP, ATX I, ATX II, AOH-3-glucoside (AOH-3-G), AOH-9-glucoside (AOH-9-G), AME-3glucoside (AME-3-G), AOH-3-S, and AME-3-sulfate (AME-3-S) (see Fig. 1) during the single processing steps, and determined the transfer rates from barley malt grist into by-products and in the final product beer. This aims to assess critical processing steps or by-products and contributes to establish regulatory thresholds to minimize the risk of toxic substances to human health.
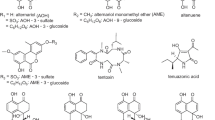
Chemical structures of the most frequent Alternaria toxins alternariol (AOH), AOH-3-glucoside (AOH-3-G), AOH-9-glucoside (AOH-9-G), AOH-3-sulfate (AOH-3-S), alternariol monomethyl ether (AME), AME-3-glucoside (AME-3-G), AME-3-sulfate (AME-3-S), alterperylenol (ALTP), altertoxin I (ATX I) and II (ATX II), stemphyltoxin III (STE III), tenuazonic acid (TeA) and tentoxin (TEN)
Materials and methods
Chemicals and reagents
Cetyltrimethylammonium bromide (CTAB) and sodium chloride were purchased from Carl Roth, Karlsruhe, Germany, tris-(hydroxymethyl)-aminomethane (Tris) and ethanol from VWR, Radnor, Pennsylvania, USA. Polyvinylpyrrolidone (PVP-40) and isoamyl alcohol were obtained from SIGMA-ALDRICH, St. Louis, Missouri, USA. Titriplex III (EDTA) was purchased from Merck, Darmstadt, Germany, Chloroform was obtained from Th. Geyer, Renningen, Germany, RNase A solution was from New England Biolabs, Ipswich, Massachusetts, USA and GreenMasterMix from Genaxxon bioscience, Ulm, Germany.
For mycotoxin analysis, methanol was purchased from Honeywell Riedel-de Haën (Seelze, Germany), water from Th. Geyer (Renningen, Germany), and acetonitrile, cyclohexane, ammonium formate and ammonia solution (25%) were obtained from VWR (Ismaning, Germany), all in analytical grade. Standards for TEN, ALT and TeA were bought from Merck (Darmstadt, Germany), and AOH, AME, ATX I, ATX II, ALTP, AOH-3-G, AOH-9-G, AME-3-G, AOH-3-S and AME-3-S were isolated from fungal culture or synthesized in our laboratory as described previously [20, 23, 24]. The stable isotope-labeled standards for [13C615N]-TeA, [2H4]-AOH and [2H4]-AME were synthesized as published before [25, 26].
Raw materials
Brewing experiments were conducted with two different naturally infected barley malt practice samples (malt 1 and malt 2), each separated into a “clean” and a contaminated “black” fraction using an optical sorting machine (SORTEX, Bühler AG, Uzwil, Switzerland). In visual sorting, contaminated kernels are recognized by their visual symptoms, typically black or red discoloration. The system was calibrated with 200 g of red and black grains, respectively, and subsequently 50 kg of malt 1 and malt 2 were completely sorted. The fractions obtained were separated for further processing using a sample divider (Pfeuffer GmbH, Kitzingen, Germany) and specified by the Mitteleuropäische Brautechnische Analysenkommission (S-590.35.001 [2013–02]) [27].
Brewing trials
Brewing experiments with a defined dosage of contaminated black kernels were performed on a semi-industrial scale in an 8 L microscale brewhouse at the Chair of Brewing and Beverage Technology (Technical University of Munich) to investigate the impact of black-colored grains on the mycotoxin levels during the different processing steps into the final bottle. To ensure a homogeneous distribution of mycotoxins (and a good reproducibility), the clean and black fractions were well-blended in the ratios 100/0, 50/50, 25/75 (clean/black, malt 1), and 100/0, 75/25 and 50/50 (clean/black, malt 2), and ground in a two-roll mill using a 0.8 mm gap. 1.5 kg grist was mashed in with 5.5 L standardized brewing water, at a malt liquor ratio of 1:3.67. The first rest was held at 62 °C for 30 min, the second rest took another 30 min at 72 °C; afterwards, the temperature was raised to 78 °C and held for 10 min. The mash was transferred to a preheated lauter tun. Trub wort pumping was performed until EBC < 35 was reached; about 3.2 L of first wort was obtained, followed by two sparges of 2.2 L water each. After lautering, the wort was boiled for 60 min at atmospheric pressure. CO2 extract of the hop variety Perle-Extrakt (43.6% α-acids) was added at the beginning of the boiling to reach 25 international bitter units (BU). After the original extract of 11.5°P was reached, hot trub was removed in a whirlpool followed by wort cooling. For fermentation, dry yeast (TUM 34/70) (Fermentis, Marcq-en-Barœul, France) was rehydrated in first wort and pitched at a concentration of 15 × 106 cells/mL. Main fermentation temperature was at 11 °C until the extract content fell below 3.5°P and followed by maturation at 16 °C until the diacetyl content was below 0.12 mg/L. The green beer was stored at 1 °C for 4 weeks. After cold storage, the beer was filtered using Seitz K150 filtration sheets (Pall-Seitz, Bad Kreuznach, Germany) and bottled oxygen free in brown glass bottles with a volume of 0.33 L. All brews were performed in duplicates, resulting in 12 separate beers.
Sampling
Samples were taken during each key step of the brewing process, including barley malt grist, mashing procedure (beginning and end), spent grains, front and original wort, hot break, yeast sediment of the main fermentation and the final filtered beer (Fig. 2). Every sampling step was thoroughly weighted to allow the calculation of a mycotoxin balance throughout the brewing process.
Sample preparation—isolation of genomic DNA from barley malt and grist
Genomic DNA from the grist mixtures and the pure clean and black malt fractions was extracted according to the CTAB-based DNA extraction protocol recommended by the European Community Reference Laboratories (European Commission CRLVL04/05XP) [28] with modifications published by Linkmeyer et al. [29]. In brief, three biological replicas were prepared per samples. Barley malt was initially ground using a Tissue Lyser II bead mill (Qiagen, Düsseldorf, Germany). 2 g of fine grain powder was mixed with 10 mL pre-warmed (65 °C) extraction buffer (2% (w/v) CTAB, 100 mM Tris pH 8, 20 mM EDTA pH 8, 1.4 M NaCl, 1% (w/v) PVP-40) and incubated for 10 min at 65 °C by gentle shaking. The samples were centrifuged at 4000 rpm for 10 min at room temperature and 1000 μL of the supernatant was transferred to a 2 mL reaction tube with 900 μL CIA 24:1 (chloroform:isoamylalcohol). Mixed by slowly inverting 20–30 times and centrifuged at 13,000 rpm for 10 min, 850 μL of supernatant was transferred into a new reaction tube, together with 8 μL RNase A solution (10 mg/mL) before incubation at 37 °C for 30 min. Then, 85 μL 10% CTAB solution (10% (w/v) CTAB, 0.7 M NaCl) and 900 μL CIA were added, followed by a centrifugation step at 13,000 rpm for 10 min. 700 μL of the supernatant was transferred to a new tube and inverted together with 70 μL 10% CTAB solution and 750 μL CIA. This was followed by centrifugation under the previous conditions. 500 μL of the upper layer was transferred into a fresh tube with 3 volumes of CTAB precipitation buffer (1% (w/v) CTAB, 0.05 M Tris, pH 8, 0.01 M EDTA pH 8) and incubated at RT for 15 min. After another centrifugation for 15 min at 13,000 rpm, the supernatant was discarded, the pellet was washed twice in 70% ethanol, air dried and dissolved in 150 μL double distilled water. The samples were stored overnight at 4 °C. The next day, the samples were centrifuged for 10 min at 13,000 rpm and 4 °C and the supernatant was transferred into a new reaction tube. The amount of DNA was determined using NanoDrop™2000c UV–Vis spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and the DNA was adjusted to a final concentration of 100 ng/μL.
Quantification of A. alternata DNA in barley malt
Genomic DNA of A. alternata in barley malts and mixed grists was detected by qPCR, as recently published [10]. In short, DNA amplification was carried out in a final reaction volume of 12 μL, containing 1X GreenMasterMix, 0.1 μM each of forward and reverse primer and 100 ng genomic DNA. qPCR was performed using a LightCycler 480ll (Roche, Basel, Switzerland), with an initial denaturation step at 95 °C for 15 min, followed by 40 cycles of 95 °C for 20 s and 60 °C/65 °C for 60 s. All samples were measured in three replicates. Subsequently A. alternata DNA was normalized according to barley DNA content and the grain infection rates were calculated as pg fungal DNA per ng plant DNA.
Preparation of stock solutions
Stock solutions of every toxin were prepared in a concentration range between 1 and 100 µg/mL and stored at − 18 °C. The toxins AOH, AME, TeA, AOH-3-S and AME-3-S were dissolved in methanol, while all other toxins were dissolved in acetonitrile. Concentrations of diluted standards were regularly controlled by UV spectrophotometric measurements (Genesys, 10S, UV–Vis spectrophotometer, Thermo Fisher Scientific, Madison, WI, USA) using quartz glass precision cells (1 cm layer thickness, Hellma GmbH & Co. KG, Müllheim, Germany). Concentrations were calculated using the respective molar extinction coefficients, which were either obtained from literature or determined in our laboratory as previously published [20, 24, 25, 30, 31].
Sample preparation for mycotoxin analysis
Mash samples, spent grains, yeast sediment and hot break were freeze-dried for 100 h (Christ-Alpha 1–2 LD Plus, Martin Christ Gefriertrocknungsanlagen GmbH, Germany) prior to analysis and the water content of each sample was determined. This value was then used to refer the mycotoxin content to the wet weight of all samples.
All samples were thoroughly ground, and sample preparations were performed as previously published [11, 20]. In brief, 1 g of grist, mash and spent grains, and 0.5 g of yeast sediment and hot break were weighted into a 50 mL centrifuge tube. For AOH, AME and TeA, stable isotope labelled standards and 15 mL of an acetonitrile/water mixture (84/16, v/v) were added to the samples for extraction. After three extractions, samples were centrifuged and the solvent was evaporated using a rotary evaporator (40 °C).
For the analysis of liquid samples (front wort, original wort and beer), stable isotope labelled standards for TeA, AOH and AME were added to 5 mL of the degassed sample. After extraction with 2.5 mL cyclohexane and a matrix precipitation with ACN, samples were centrifuged and supernatants were evaporated using a rotary evaporator (40 °C).
Afterwards, both solid and liquid sample extracts were further cleaned up using solid phase extraction as described previously. The eluate was evaporated, again, and the residue was taken up in 1 mL acetonitrile/water (3/7, v/v). Samples were then membrane filtered using a 0.22 µm PVDF filter and stored at − 18 °C until LC–MS/MS measurements.
LC–MS/MS analysis
All samples were measured with a previously published LC–MS/MS method for the analysis of 13 free and modified Alternaria toxins [11, 20].
In brief, analysis was performed on a Shimadzu Nexera X2 UHPLC system (Shimadzu, Kyoto, Japan). For tenuazonic acid, a Gemini-NX C18 column (150 × 4.6 mm, 3 µm, 110 Å, Phenomenex, Aschaffenburg, Germany) was used as published before, all other toxins were separated on a Hyperclone BDS C18 column (150 × 3.2 mm, 3 µm, 130 Å, Phenomenex, Aschaffenburg, Germany). The HPLC system provided automated column switching and fourfold solvent selection for each pump, and both methods could be run in sequence.
The LC was interfaced with a Shimadzu 8050 triple quadrupole mass spectrometer (Shimadzu Corporation, Kyoto, Japan) and measurements were conducted in the negative electrospray ionisation (ESI) mode. All MS/MS measurements were operated in the multiple reaction monitoring (MRM) mode. For data acquisition and data analysis, the LabSolutions Software (Shimadzu, Kyoto, Japan) was used.
The limits of detection (LOD) and the limits of quantitation (LOQ) for both solid and liquid samples are summarized in Table 1.
Results and discussion
Quantification of A. alternata DNA in the raw material
Brewing was conducted with two different malt samples, each optically sorted into visually clean and black grains and remixed in different ratios as described above. To determine the mycological status of the used raw materials, genomic DNA from the respective fractions was extracted and the fungal infection levels (pg fungal DNA per ng barley DNA) were calculated. The two malt samples used in this study differed widely in their fungal DNA concentration, which was reflected in both the purified (0% black grains) and the discarded colored fraction (100% black grains). The respective data can be found in Table 2. Malt sample 1 was heavily loaded with A. alternata DNA in the clean fraction (19.65 pg/ng and 31.21 pg/ng) and even higher in the black-colored fraction (36.21 pg/ng). For malt 2, contamination was significantly lower at 8.83 pg/ng and 7.25 pg/ng in the clean fraction and, interestingly, even lower at 4.36 pg/ng in the black-colored fraction. Thus, the two practical samples represent a batch heavily contaminated with A. alternata, as appeared in 2021 on one hand, and a moderately contaminated sample on the other hand [10].
Quantification of Alternaria toxins in the raw material
The analysis of mycotoxins in the grist has shown the presence of AOH, AME, TeA, TEN, ALTP and ATX I in all used ratios of malt 1, while AOH-3-S was only detected in the grist sample with 75% black grains (see supplementary information, Tables S1–4). In the samples with 0% black grains, the concentrations of AOH and ATX I were below the limit of quantitation (LOQ), while concentrations in the samples with 50% or 75% black grains were elevated for all toxins compared to the batch with 0% black grains. For malt 2, only AOH and TeA were detected in the grist samples of all mixtures with AOH showing concentrations below the LOQ for all ratios, while TeA values were comparable for all 6 samples (see Supplementary Information, Table S5). The toxins ATX II, ALT, AOH-9-G, AME-3-S and AME-3-G were not detected in any grist sample.
As Alternaria spp. is ubiquitously present, it was not expected to obtain a completely mycotoxin-free batch by optical sorting. However, mycotoxin analyses of the grist have already shown an impact of removing the black grains as only low mycotoxin contents were found afterwards. For the DNA results, cleaning also resulted in lower Alternaria levels for malt 1, while no difference in DNA contents could be observed in malt 2, which makes the evolution of mycotoxins in these samples even more interesting.
Evolution of Alternaria toxins during brewing
Hops and yeasts added during brewing were not analyzed separately, since the applied methods were not suitable for hop analysis. However, according to the literature, no mycotoxins should be present there [32] and no increase in the mycotoxin content was observed in the following sampling steps. In addition, in case of toxin contamination of the hop, the amount of added hop extract (0.05–0.25%) should only contribute neglectable amounts of mycotoxins to the final product. The same accounts for yeast (0.5%) [3]. Consequently, detected toxins should, therefore, be deriving from the mycotoxin load of the used grist. To calculate the mycotoxin balance, the values detected in the grist were set to 100%, and for concentrations below the LOQ [11, 20], a value of ½ x LOQ was used for further calculations of the mycotoxin balance. This so-called middle-bound (MB) scenario is one potential way to handle left-censored data that often occur in mycotoxin research. For example, EFSA used this approach in their exposure assessment to Alternaria toxins [33]. The MB scenario provides a more realistic approach than the lower bound (LB) scenario (assigning a value of zero to samples below LOD and LOQ) and was used in this study, therefore. However, calculating the mycotoxin balance with values below the LOQ can misrepresent the quantitative transfer of toxins due to analytical uncertainties and, consequently, some detected toxins in this work were interpreted only indicatively.
According to the recently published validation data for the detected toxins [11, 20], the method used for liquid samples had lower limits of detections for AOH, AME, TeA and ATX I, while the values for TEN, ALTP and AOH-3-S were comparable for both matrices (see Table 1). This means that the analysis of these three toxins is more impacted by dilution effects in the liquid sampling steps than the other toxins. In addition, recovery values of AOH, AME, TEN, ALTP and AOH-3-S were comparable for both methods, while TeA showed a slightly higher recovery and ATX I a slightly lower recovery in solid samples compared to liquid samples. Consequently, only minor, negligible errors should be present in the mycotoxin balance due to different sample preparations of solid and liquid samples. In the following, the evolution of mycotoxins during brewing are discussed individually or according to their structural groups. The ALT, ATX II, AOH-9-G and AME-3-G toxins were not detected in any sample and, consequently, are not further discussed.
Tenuazonic acid
In this study, TeA was the only toxin to be found to fully migrate into the final beer. In malt 1 (see Fig. 3), a decrease of concentration was observed at the beginning of the mash, whereas an increase of up to 157% of the initial content in the grist could be observed in four of the six brews at the end of mashing. We assume that due to the higher temperature and possibly also to further enzymatic processes, matrix-bound TeA might be released resulting in a higher concentration after mashing. However, bound Alternaria toxins are not known yet, but have been found before for other mycotoxins [34]. Hence, further studies are necessary to proof this theory. After lautering, only 15–22% of the initial TeA concentration could be found in the spent grains, while 90–228% were detected in the first wort. TeA contents did not change in the original wort, and after a loss of 11–20% in the hot break, and a loss of 4–17% in the yeast sediment, about 27–67% of the initially present TeA was detected in the filtered beer samples.
Evolution of the Alternaria toxins AOH, AME, ALTP, TeA, TEN and ATX I during the brewing process using malt sample 1 with a 0% black grains, b 50% black grains and c 75% black grains. Every mixture was brewed in duplicate (R1 = Replicate 1, R2 = Replicate 2). The toxin content in the grist was set to 100%, detailed values can be found in the Supplementary Information
For TeA in malt 2 (see Fig. 4), the obtained results were also comparable to malt 1, even though overall concentrations were lower than in malt 1. During mashing, concentrations of TeA decreased compared to the amounts in the grist at the beginning, while an increase could be observed once again at the end of mashing. At the beginning of mashing, one brew showed elevated TeA concentrations compared to the grist and the end of mashing. Hence, we assume that the obtained sample of the beginning of mashing might not have been representative for the whole batch. Regarding the spent grains, only 6–30% of the initial TeA content could be found in this step, which agrees with the observations we made for malt 1. In addition, 51–176% of the TeA content was detected in the first wort, which stayed constant in the original wort. With losses of 30–115% of the initial concentration in the hot break, and an additional loss of 6–15% in the yeast sediment, the amount of TeA found in the filtered beer was 16–70% of the amount present in the grist. As before, the sum of TeA found in the spent grains, hot break, yeast sediment and the filtered beer was calculated for every beer and summarized in Fig. 5. Finally, we calculated the sum of TeA found in the spent grains, hot break, yeast sediment and the final beer for all brews, resulting in values of 53–120% and 61–193% (malt 1 and malt 2, respectively) compared to the concentration found in the grist (see Fig. 5).
Evolution of the Alternaria toxins AOH and TeA during the brewing process using malt sample 2 with a 0% black grains, b 25% black grains and c 50% black grains. Every mixture was brewed in duplicate (R1 = Replicate 1, R2 = Replicate 2). The toxin content in the grist was set to 100%, detailed values can be found in the Supplementary Information. Alternaria toxins that are not displayed in the figure could not be detected in any sample
Percentage comparison of the initial TeA content and its distribution throughout the brewing process shown as the sum of the percentages found in the spent grains, hot break, yeast sediment and the final beer, respectively for a malt 1 and b malt 2 with the respective proportion of black grains in the starting material in %
Therefore, it can be concluded that TeA can be formed or released during mashing and is then only partly removed throughout the brewing process.
Dibenzo-α-pyrones
The dibenzo-α-pyrones AOH and AME were found in several samples of malt 1 during brewing (see Fig. 3), and could be quantified in all samples except the brew with 0% black grains, where AOH was below the LOQ in almost all samples, and in the wort samples of the other two mixtures. For the mixtures with 50% and 75%, concentrations of AOH stayed relatively stable at beginning of mashing compared to the initial levels. Regarding AME, a decrease of up to about 70% in one and about 40% in three samples could be found. Here, the metabolite AME-3-S was detected during mashing, which partially explains the reduction of AME content in this processing step. At the end of mashing, an increase of AOH and AME of about 140–170% and 120–170%, respectively, was observed in two brews (0% black grains).The contents of both toxins in the other four brews were relatively stable compared to the beginning of mashing. After lautering, between about 160% and 380% of the initial AOH content, and between about 100% and 360% of the initial AME content could be found in the spent grains, showing an accumulation of mycotoxins in this fraction. This transfer into the spent grains was also shown by Prusova et al. [6], although they did not notice increasing mycotoxin concentrations. This might be due to overall higher concentrations in their study that are less affected by analytical uncertainties in the low concentration range like in this work. However, they did observe the transfer of small amounts of AOH and AME into the wort as well, which was also the case in our study. In malt 2 (see Fig. 4), data for AOH is hard to compare as concentrations in the grist and mash (beginning and end) were below the LOQ in all mixtures, and therefore, no changes could be observed. However, the AOH content found in the spent grains was two- to threefold higher than the initial amount, which is identical to the observations we made in malt 1 and shows that this toxin is also most likely to be removed from the brewing process through the spent grains.
Perylenequinones
The perylenequinones ALTP and ATX I (see Fig. 3) were only present in the grist samples, during mashing, and in the spent grains of malt 1, but were not found in malt 2. For ALTP, only one grist sample (50% black grains) was below the LOQ, which added uncertainty to the calculation of the balance, and therefore, results of this brew will not be further discussed. For the other brews, 44–99% of the initial content of the grist could be found at the beginning of mashing. Afterwards, ALTP contents stayed stable or only increased slightly during mashing, and almost all ALTP found in the mash was also observed in the spent grains, meaning that ALTP is most likely to be removed from the brewing process through this brewery by-product.
For ATX I, values below the LOQ were found in three of the six brews, which only allowed an indicative interpretation. Increased contents up to about 200% were found at the beginning of the mash in the other brews, however, at the end of the mashing process, we noticed differing patterns as both increased (in the 0% mixture) and decreased concentrations (in the 50% mixture) as well as no ATX I at all (in the 75% mixture) were found in two brews, respectively, which means that ATX I was mainly found in the clean and not in the black grains. For the brews with 75% black grains, ATX I could be observed below the LOQ in the spent grains, which let us assume that ATX I was present at the end of mashing as well, but below the limit of detection (LOD) due to measurement uncertainty. As for the other toxins discussed so far, ATX I seems to be entirely removed from the brewing process with the spent grains, and no ATX I could be detected in any of the following processing steps. To our knowledge, this is the first time that ALTP and ATX I were analyzed during brewing.
Tentoxin
Like ALTP, a decrease of TEN could be observed during the beginning of mashing compared to the grist samples (see Fig. 3). Here, only about 30–60% of the initial concentration could be observed in this processing step, and concentrations stayed stable during mashing. The TEN contents of the mash could further be found in the spent grains, showing again a reduction of Alternaria toxins during brewing by transfer into this by-product. However, TEN could also be quantified in the first and original wort of most brews, and the sum of TEN in the wort and spent grains mainly resulted in the initial concentrations in the grist, especially when the low concentrations of TEN (often < 0.5 µg/kg) and measurement uncertainty are considered. This shows that the lower concentrations found in mash samples might be attributed to transfer into the supernatant water that was removed from the sample before analysis as TEN was found to be highly water soluble. However, after the original wort, no TEN could be further detected, which might be due to the dilution effects or concentrations below or close the LOD. These observations are also comparable to the study from Prusova et al. [6], where 87% of the initial present TEN content was transferred into the wort, and low concentrations could be found in the spent grains.
Modified mycotoxins
The modified Alternaria toxins AOH-3-S, AME-3-S and AOH-3-G were only rarely found in malt 1, and not detected in malt 2 (see Supplement Information, Table S4). AOH-3-S could be detected at the beginning of mashing in the samples containing 75% black grains in an 8- to tenfold amount compared to the grist, while no AOH-3-S could be detected in the other four brews of malt 1. A further increase of AOH-3-S was observed at the end of mashing in one brew (replicate 1, 75% black grains), but concentrations in the other brew (replicate 2, 75% black grains) were slightly lower than before. In the spent grains, AOH-3-S was only found in concentrations below the LOQ. Here, the fate of the formed amounts of AOH-3-S during mashing is unclear and needs further investigation in the future. In addition, AOH-3-G was found in two grist samples as well as in four samples at the beginning of mashing (both in the mixtures 50% and 75% black grains), and AME-3-S could be detected in both the mash and the spent grains in all brews of malt 1 containing 50% and 75% black grains. Unfortunately, concentrations of both toxins were below the LOQ in all processing steps, which makes further interpretation about their formation unfeasible.
In summary, we could demonstrate the transfer of the highly polar Alternaria toxin TeA into the final beer, which is consistent with previous research [6]. As TeA is known to be the most dominant toxin in malt and is often present in higher concentrations than the other Alternaria toxins [11], the observed results of this study were expected, especially as several studies have already shown the presence of TeA in commercial beer samples [18,19,20]. However, TeA concentrations in the beer brewed with 75% black kernels (malt 1) showed slightly higher concentrations than the majority of beers analyzed in previous surveys [18,19,20]. AOH and AME were also regularly detected in beer before [17, 18], which was not the case in this study. Nevertheless, we could observe the transfer of AOH and AME into the wort, which shows the overall ability of both toxins to migrate through the brewing process, and the absence in the final beer might be due to the low concentrations in the used malt samples. Therefore, we assume that both AOH and AME, and probably also TEN, are likely to be found in beer if concentrations in the used grist are higher than in our study. However, the concentrations found in the grist were comparable to the results of a previously published study of Alternaria toxins in 50 barley and malt samples from Germany [11], and therefore, no significantly higher toxin concentrations should be expected in malt. In addition, Prusova et al. [6] suspected the physical adsorption of some toxins to the yeast, which might explain a reduction in toxin concentrations at this stage of the brewing process. However, no toxins except TeA were found in the analyzed yeast sediment, but this might also be due to concentrations below the LOD, and therefore, this hypothesis can only be confirmed for TeA, so far. The Alternaria toxins ALTP and ATX I are most likely to be fully removed with the spent grain, whereas the fate of the modified toxin AOH-3-S is still not clear. Here, more information on further modification processes is necessary and the use of spent grains as animal feeds should be assessed critically, if the initial raw material shows signs of Alternaria infection.
Impact of black-colored grain on mycotoxin concentrations during brewing
Brewing with varying proportions of black-colored grains were performed to gain information on the relationship between black symptomatic kernels, total fungal DNA and occurrence and levels of mycotoxins. The references that were brewed exclusively with optically clean grains of malt 1, showed the lowest mycotoxin concentrations in almost all cases, while samples from brewing with 75% black grains contained the highest amounts of mycotoxins (see Supplementary Information, Tables S6 and S7). Consequently, this study shows that the presence of mycotoxins was in line with the addition of black grains in most cases. However, the ratio of black and clean kernels was not fully reflected as the mycotoxin content in the 50% and 75% mixture brews differed without a visible pattern and were similar in several sampling steps. Consequently, a prediction of mycotoxin concentration for other ratios of clean and black kernels cannot be reasonably performed. These findings are consistent with DNA levels (Table 2), as the separated black fraction had higher A. alternata DNA values than the optically clean fraction. However, although Alternaria spp. DNA levels increased with the proportion of black grains in the mixed grists, the mixing ratio was not fully reflected.
For malt 2, the concentrations of AOH and TeA did not indicate any trends regarding the amount of used black kernels and were comparable for all brews and sampling steps showing no impact of the black kernels on the mycotoxin levels. This was also the case for the DNA analyses, which showed no differences for the varying blends 0, 25 and 50% mixture, respectively. Nevertheless, the lowest and highest TeA concentration of each brew were detected in the 0 and 50% mixture, respectively. We, therefore, conclude that optical sorting might still be a suitable tool for the malt and brewing industries to reduce mycotoxin levels in their final products. From an economic point of view, sorting the raw barley before the malting process would be more obvious. However, in our previous study we showed that more malt samples than barley samples revealed contamination with Alternaria toxins, which indicates a mycotoxin formation during the malting process. Therefore, we see the optical sorting as an additional way to subsequently improve a batch regarding food safety.
Nevertheless, the experiments with malt 2 revealed, that dark kernel symptomatology is not always a reliable indicator of A. alternata fungal infestation and probably has other origins. In the literature, three main forms of kernel discoloration are commonly described: first, weather staining can be responsible for a colour change of the kernel from light to deep yellow or a caramelized colour, which is often described as grain tan [35]. Second, a black–brown discoloration at the embryo end of the grain is known as germ end staining or black point disease, which is commonly associated with fungal infection, but was also interpreted as result of enzymatic browning reactions [35, 36]. Finally, in extreme cases, a greyish hue or distinctive black spots can be caused by visible mould formation [35]. However, it is also assumed that several different causative factors might induce grain discoloration [37]. One widely accepted hypothesis is that black point formation is a result of biochemical changes when exposed to environmental effects, which then lead to enzymatic browning [38, 39]. This is commonly known as a characteristic reaction of plants that are subjected to stressful conditions, and takes place by the oxidation of phenolic compounds, which then form brown or black pigments known as melanins [36, 40].
In general, A. alternata is suspected to be the main causal agent for black spots on wheat and barley grains since many years [16, 36, 38, 41], but other fungi such as Cochliobolus sativus, Fusarium spp., Stemphylium spp., Cladosporium spp. or Epicoccum spp. may also be able to create similar kernel discoloration patterns [36, 41]. However, until now, studies only focused on fungal isolations and not on mycotoxin concentrations. In contrast, there are also many reports, where no evidence of an association between fungal infections and black spots was found [42,43,44]. As shown in a previous study [10], the dark kernel symptomatology has no significant relationship with DNA content with respect to the fungus A. alternata. In this study, the mycotoxin content was added as a new factor and statistical analysis between A. alternata DNA levels and the individual toxin content based on the two-tailed t test (data not shown), revealed a strong positive directional correlation only by the toxin ALTP (malt 1), which was found to be significant (ALTP: r = 0.862, p ≤ 0.05). In malt 2, a very strong negative directional correlation was shown for both mycotoxins detected in the raw material (AOH and TeA), which proved to be significant in this case (AOH: r = − 0.911, p ≤ 0.05; TeA: r = -0.902, p ≤ 0.05). However, no significant relationship was found for the other toxins, but further investigations are needed with a bigger sample set. For now, this preliminary study is the first time the correlation between A. alternata DNA levels and Alternaria toxin concentration was calculated. In addition, other Alternaria species besides A. alternata could be present on the raw material as well and be responsible for the detected toxins. Hence, DNA analyses of these species should be included in future projects.
Conclusion
Knowledge of the distribution of Alternaria mycotoxins during the brewing process enables assessing critical processing steps or by-products, and will help to assess the need of regulatory thresholds to minimize the risk of toxic substances to human health. In this study, brewing trials with defined dosages of contaminated black kernels were successfully conducted to investigate the fate of Alternaria mycotoxins in the brewing process depending on the initial contamination and, based on this, to determine relationships between black symptomatic kernels, total fungal DNA, and levels of mycotoxins.
Quantification of A. alternata DNA in the raw material revealed higher levels in the black-colored fraction of malt sample 1, compared to the clean reference, with fungal DNA values increasing with the proportion of black grains. This could not be confirmed for malt sample 2, as for this sample the DNA values of the black-colored fraction were comparable with the DNA content in the respective mixtures. The statistical analysis did not show any significant correlation between Alternaria mycotoxins and A. alternata DNA content, except for ALTP I, and occasionally AOH and TeA. However, the verification of this correlation needs further investigation. Therefore, toxin production, which belongs to the secondary metabolism of the fungus, does not necessarily have to be linked to fungal biomass (= primary metabolism). Nevertheless, higher mycotoxin concentrations were found during the brewing experiments with malt 1, when 50 or 75% black grains were used compared to the brews with clean grains.
TeA was the only toxin to migrate into the final beer due to its high polarity, while the AOH, AME, TEN, ALTP and ATX I toxins were mainly found in the spent grains. A further transfer of AOH and AME into the wort could be observed, which shows the overall ability of both toxins to migrate through the brewing process. The observance of AOH-3-S and AME-3-S in some sampling steps also demonstrated the possibility of modification reactions during brewing. AOH-3-S was only detectable in samples from the 75% mixture brew, which could probably show that higher AOH concentrations are necessary before the sulfates are formed. The ATX II, ALT, AOH-9-G and AME-3-G toxins were not detected in any sample.
Furthermore, the results strongly indicate that optical sorting is a suitable tool for the malting and brewing industries to reduce mycotoxin levels in their final products. Even without black discoloration being necessarily an infection with Alternaria spp., this method might be useful to decrease economical losses in the barley industry and should, therefore, be further optimized. Kernel discoloration is often associated with a high microbial load, which then leads to substantial economic losses due to downgrading of raw materials to animal feed [41, 45, 46]. Nevertheless, screening for Alternaria DNA and/or mycotoxin levels will still remain important as quality control in the future, as non-discolored cereals can still be heavily infected with this fungus and its toxins [37, 44, 47].
The relationship between A. alternata DNA and the respective Alternaria mycotoxins should be investigated in more detail in the future to improve food safety. Further studies are also required to gain more insight into the modification processes of AOH-3-S, as its fate could not be elucidated in this study. In addition, the relationship between AOH concentrations and the formation of the sulfates is a suggestion for future research approaches, as is the study of matrix-bound Alternaria toxins, which are not yet known, but have been found before for other mycotoxins.
Data availability
All data to support the findings of the manuscript are presented in the main document and the supporting information.
References
Justé A (2011) Microflora during malting of barley: overview and impact on malt quality. Brewing Science 64:22–31
Walling JG, Zalapa LA, Vinje MA (2018) Evaluation and selection of internal reference genes from two- and six-row U.S. malting barley varieties throughout micromalting for use in RT-qPCR. PLoS One. https://doi.org/10.1371/journal.pone.0196966
Narziß L, Back W, Gastl M, Zarnkow M. Abriss der Bierbrauerei. 8., vollständig überarbeitete und erweiterte Auflage. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2017.
Hu L, Gastl M, Linkmeyer A, Hess M, Rychlik M (2014) Fate of enniatins and beauvericin during the malting and brewing process determined by stable isotope dilution assays. LWT. https://doi.org/10.1016/j.lwt.2013.11.004
European Food Safety Authority (EFSA). scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA Journal. 2011; https://doi.org/10.2903/j.efsa.2011.2407
Prusova N, Dzuman Z, Jelinek L, Karabin M, Hajslova J, Rychlik M, Stranska M (2021) Free and conjugated Alternaria and Fusarium mycotoxins during pilsner malt production and double-mash brewing. Food Chem. https://doi.org/10.1016/j.foodchem.2021.130926
Lancova K, Hajslova J, Poustka J, Krplova A, Zachariasova M, Dostalek P, Sachambula L (2008) Transfer of Fusarium mycotoxins and “masked” deoxynivalenol (deoxynivalenol-3-glucoside) from field barley through malt to beer. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. https://doi.org/10.1080/02652030701779625
Habler K, Geissinger C, Hofer K, Schüler J, Moghari S, Hess M, Gastl M, Rychlik M (2017) Fate of Fusarium toxins during brewing. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.6b04182
Piacentini KC, Benešová K, Pernica M, Savi GD, Rocha LO, Hartman I, Čáslavský J, Corrêa B, Běláková S (2019) Fusarium mycotoxins stability during the malting and brewing processes. Toxins (Basel). https://doi.org/10.3390/toxins11050257
Bretträger M, Becker T, Gastl M. Screening of mycotoxigenic fungi in barley and barley malt (Hordeum vulgare L.) using real-time PCR—A comparison between molecular diagnostic and culture technique. Foods. 2022; https://doi.org/10.3390/foods11081149
Scheibenzuber S, Dick F, Bretträger M, Gastl M, Asam S, Rychlik M (2022) Development of analytical methods to study the effect of malting on levels of free and modified forms of Alternaria mycotoxins in barley. Mycotoxin Res. https://doi.org/10.1007/s12550-022-00455-1
Noots I, Delcour JA, Michiels CW (1999) From field barley to malt: detection and specification of microbial activity for quality aspects. Crit Rev Microbiol. https://doi.org/10.1080/10408419991299257
Krasauskas A (2017) Fungi in malting barley grain and malt production. Biologija. https://doi.org/10.6001/biologija.v63i3.3583
Felšöciová S, Kowalczewski PŁ, Krajčovič T, Dráb Š, Kačániová M (2021) Effect of long-term storage on mycobiota of barley grain and malt. Plants (Basel). https://doi.org/10.3390/plants10081655
Logrieco A, Moretti A, Solfrizzo M (2009) Alternaria toxins and plant diseases: an overview of origin, occurrence and risks. World Mycotoxin J. https://doi.org/10.3920/WMJ2009.1145
Amatulli MT, Fanelli F, Moretti A, Mule G, Logrieco AF (2013) Alternaria species and mycotoxins associated to black point of cereals. Mycotoxins. https://doi.org/10.2520/myco.63.39
Bauer JI, Gross M, Gottschalk C, Usleber E (2016) Investigations on the occurrence of mycotoxins in beer. Food Control. https://doi.org/10.1016/j.foodcont.2015.11.040
Prelle A, Spadaro D, Garibaldi A, Gullino ML (2013) A new method for detection of five Alternaria toxins in food matrices based on LC-APCI-MS. Food Chem. https://doi.org/10.1016/j.foodchem.2012.12.065
Siegel D, Merkel S, Koch M, Nehls I (2010) Quantification of the Alternaria mycotoxin tenuazonic acid in beer. Food Chem. https://doi.org/10.1016/j.foodchem.2009.10.070
Scheibenzuber S, Dick F, Asam S, Rychlik M (2021) Analysis of 13 Alternaria mycotoxins including modified forms in beer. Mycotoxin Res. https://doi.org/10.1007/s12550-021-00424-0
Femenias A, Gatius F, Ramos AJ, Teixido-Orries I, Marín S (2022) Hyperspectral imaging for the classification of individual cereal kernels according to fungal and mycotoxins contamination: a review. Food Res Int. https://doi.org/10.1016/j.foodres.2022.111102
Pascale M, Logrieco AF, Graeber M, Hirschberger M, Reichel M, Lippolis V, De Girolamo A, Lattanzio VMT, Slettengren K (2020) Aflatoxin reduction in maize by industrial-scale cleaning solutions. Toxins. https://doi.org/10.3390/toxins12050331
Gotthardt M, Kanawati B, Schmidt F, Asam S, Hammerl R, Frank O, Hofmann T, Schmitt-Kopplin P, Rychlik M (2020) Comprehensive analysis of the Alternaria mycobolome using mass spectrometry based metabolomics. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201900558
Scheibenzuber S, Hoffmann T, Effenberger I, Schwab W, Asam S, Rychlik M (2020) Enzymatic synthesis of modified Alternaria mycotoxins using a whole-cell biotransformation system. Toxins (Basel). https://doi.org/10.3390/toxins12040264
Asam S, Konitzer K, Schieberle P, Rychlik M (2009) Stable isotope dilution assays of alternariol and alternariol monomethyl ether in beverages. J Agric Food Chem. https://doi.org/10.1021/jf900450w
Asam S, Liu Y, Konitzer K, Rychlik M (2011) Development of a stable isotope dilution assay for tenuazonic acid. J Agric Food Chem. https://doi.org/10.1021/jf104270e
MEBAK online. Methode S-590.35.001. Probennahme und Probenteilung. Rev. 2013-02. Mitteleuropäische Brautechnische Analysenkommission (MEBAK®) e.V., Freising. https://www.mebak.org/methode/s-590-35-001/probennahme-und-probenteilung/2376. Accesed on 13 Mar 2023
Joint Research Centre. Maize Seed Sampling and DNA Extraction: Document CRLV04/05XP. 2007.
Linkmeyer A, Götz M, Hu L, Asam S, Rychlik M, Hausladen H, Hess M, Hückelhoven R (2013) Assessment and introduction of quantitative resistance to Fusarium head blight in elite spring barley. Phytopathology. https://doi.org/10.1094/PHYTO-02-13-0056-R
Cole RJ, Schweikert MA, Jarvis BB (2003) Handbook of secondary fungal metabolites. Academic, Oxford
Fleck SC. In vitro-Studien zum genotoxischen Potential und zum Metabolismus von Mykotoxinen mit Resorcylsäurelakton- und Perylenchinon-Struktur. Zugl.: Karlsruher Institut für Technologie, KIT, Diss., 2013. Karlsruhe, Baden: KIT Scientific Publishing; 2014.
Scott PM (1996) Mycotoxins transmitted into beer from contaminated grains during brewing. J AOAC Int 79:875–882
EFSA (2016) (European Food Safety Authority), Arcella D, Eskola M and Gomez Ruiz JA. Scientific report on the dietary exposure assessment to Alternaria toxins in the European population. EFSA Journal https://doi.org/10.2903/j.efsa.2016.4654
Berthiller F, Schuhmacher R, Adam G, Krska R (2009) Formation, determination and significance of masked and other conjugated mycotoxins. Anal Bioanal Chem. https://doi.org/10.1007/s00216-009-2874-x
Young K, Loughman R (2001). Fungal associations with weather-stained barley in Western Australia. Conference Paper, 10th Australian Barley Technical Symposium.
Walker KR, Able JA, Mather DE, Able AJ (2008) Black point formation in barley: environmental influences and quantitative trait loci. Aust J Agric Res. https://doi.org/10.1071/AR08074
Marasas W, van Wyk PS, Jacobs G. Black-point of barley: colonisation of trichomes by Alternaria alternata. Phytophylactica. 1990:251–253.
Williamson PM (1997) Black point of wheat: in vitro production of symptoms, enzymes involved, and association with Alternaria alternata. Aust J Agric Res. https://doi.org/10.1071/A96068
Cochrane M (1994) Observations on the germ aleurone of barley. Phenol oxidase and peroxidase activity. Annals of Botany. https://doi.org/10.1006/anbo.1994.1014
Walker JR, Ferrar PH (1998) Diphenol oxidases, enzyme-catalysed browning and plant disease resistance. Biotechnol Genet Eng Rev. https://doi.org/10.1080/02648725.1998.10647966
Rees R, Martin DJ, Law D (1984) Black point in bread wheat: effects on quality and germination, and fungal associations. Aust J Exp Agric Anim Hus. https://doi.org/10.1071/EA9840601
Conner RL, Davidson JGN (1988) Resistance in wheat to black point caused by Alternaria alternata and Cochliobolus sativus. Can J Plant Sci. https://doi.org/10.4141/cjps88-046
Jacobs B, Rabie CJ. The correlation between mycelial presence and black-point in barley. Phytophylactica. 1987:77–81.
Ellis SA, Gooding MJ, Thompson AJ (1996) Factors influencing the relative susceptibility of wheat cultivars (Triticum aestivum L.) to blackpoint. Crop Protection. https://doi.org/10.1016/0261-2194(95)00115-8
Fernandez MR, Conner RL. Black point and smudge in wheat. Prairie Soils & Crops Journal. 2011:158–164.
Li CD, Lance RCM, Collins HM, Tarr A, Roumeliotis S, Harasymow S, Cakir M, Fox GP, Grime CR, Broughton S, Young KJ, Raman H, Barr AR, Moody DB, Read BJ (2003) Quantitative trait loci controlling kernel discoloration in barley (Hordeum vulgare L). Aust J Agric Res. https://doi.org/10.1071/ar03002
Roháčik T, Hudec K (2008) Fungal infection of malt barley kernels in slovak republic. Plant Protect Sci. https://doi.org/10.17221/2249-PPS
Acknowledgements
The authors would like to thank Bühler AG (Uzwil, Switzerland) for providing the equipment for processing the samples.
Funding
Open Access funding enabled and organized by Projekt DEAL. The research leading to these results was funded by the IGF Project (AiF 19766 N) of the FEI, supported via AiF within the program for promoting the Industrial Collective Research (IGF) of the Federal Ministry of Economic Affairs and Climate Action (BMWK), based on a resolution of the German Parliament. This study was supported by the Forschungskreis der Ernährungsindustrie e.V. (FEI), (Wissenschaftsförderung der Deutschen Brauwirtschaft e.V. (WiFö) and Wissenschaftliche Station für Brauerei in München e.V.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Brewing and sampling were conducted by MB and MG, DNA analysis was performed by MB, and the mycotoxin analysis was executed by SS. The first draft of this manuscript was written by SS and MB, and all authors commented on previous versions of this manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Compliance with ethics requirements
This research does not include humans and animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bretträger, M., Scheibenzuber, S., Asam, S. et al. Evolution of Alternaria toxins during the brewing process and the usability of optical sorting methods to reduce mycotoxin concentrations in beer. Eur Food Res Technol 249, 1613–1626 (2023). https://doi.org/10.1007/s00217-023-04241-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04241-w