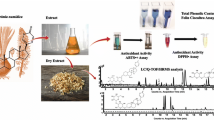
Abstract
The nasturtium (Tropaeolum majus L.) contains many biologically active compounds with very promising effects on human health. Our attention was paid to glucotropaeolin and phenolic compounds that were simultaneously determined in different parts of nasturtium using rapid reversed-phase high performance liquid chromatography coupled to tandem mass spectrometry. Mainly isomers of hydroxycinnamic acid and derivatives of quinic acid, kaempferol, and quercetin were present. Moreover, many of them were identified for the first time. Their representation varied significantly depending on the part of nasturtium (flower, stem, seed, and leaf). Although the highest total concentration of the target compounds was found in leaves, all monitored compounds were present in flowers at concentrations higher than their limit of quantification. Furthermore, the effect of sample pre-treatment (drying and freezing) on their content was investigated. Surprisingly, frozen samples showed a considerable reduction in glucotropaeolin content. Finally, antioxidant capacity, total phenolic content, and total anthocyanin content were determined using spectrophotometric techniques and the results were compared to chromatographic data.
Graphical abstract





Similar content being viewed by others
Abbreviations
- ABTS:
-
2,2 ‘-Azinobis(3-ethyl-2, 3-dihydrobenzothiazol-6-sulfonát)
- DPPH:
-
1,1 ‘-Difenyl–2-pikrylhydrazyl
- FW:
-
Fresh weight
- GAE:
-
Gallic acid equivalent
- GTL:
-
Glucotropaeolin
- PPs:
-
Phenolic compounds
- TAC:
-
Total anthocyanin content
- TEAC:
-
Trolox equivalent antioxidant capacity
- TPC:
-
Total phenolic content
References
Bazylko A, Granica S, Filipek A, Piwowarski J, Stefańska J, Osińska E, Kiss AK (2013) Comparison of antioxidant, anti-inflammatory, antimicrobial activity and chemical composition of aqueous and hydroethanolic extracts of the herb of Tropaeolum majus L. Ind Crops Prod 50:88–94. https://doi.org/10.1016/j.indcrop.2013.07.003
Brondani J, Cuelho C, Marangoni L, Lima R, Guex C, Bonilha I, Manfron M (2016) Traditional usages, botany, phytochemistry, biological activity and toxicology of Tropaeolum majus L. - A review. Bol Latinoam Caribe Plant Med Aromat 15(4):264–273
Garzón GA, Wrolstad RE (2009) Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus). Food Chem 114(1):44–49. https://doi.org/10.1016/j.foodchem.2008.09.013
Jakubczyk K, Janda K, Watychowicz K, Łukasiak J, Wolska J (2018) Garden nasturtium (Tropaeolum majus L.) - a source of mineral elements and bioactive compounds. Rocz Panstw Zakl Hig 69(2):119–126
Gasparotto Junior A, Boffo MA, Lourenço EL, Stefanello ME, Kassuya CA, Marques MC (2009) Natriuretic and diuretic effects of Tropaeolum majus (Tropaeolaceae) in rats. J Ethnopharmacol 122(3):517–522. https://doi.org/10.1016/j.jep.2009.01.021
Garzón GA, Manns DC, Riedl K, Schwartz SJ, Padilla-Zakour O (2015) Identification of phenolic compounds in petals of nasturtium flowers (Tropaeolum majus) by high-performance liquid chromatography coupled to mass spectrometry and determination of oxygen radical absorbance capacity (ORAC). J Agric Food Chem 63(6):1803–1811. https://doi.org/10.1021/jf503366c
Koike A, Barreira JCM, Barros L, Santos-Buelga C, Villavicencio ALCH, Ferreira ICFR (2015) Irradiation as a novel approach to improve quality of Tropaeolum majus L. flowers: Benefits in phenolic profiles and antioxidant activity. Innov Food Sci Emerg Technol 30:138–144. https://doi.org/10.1016/j.ifset.2015.04.009
Navarro-González I, González-Barrio R, García-Valverde V, Bautista-Ortín AB, Periago MJ (2015) Nutritional composition and antioxidant capacity in edible flowers: characterisation of phenolic compounds by HPLC-DAD-ESI/MSn. Int J Mol Sci 16(1):805–822. https://doi.org/10.3390/ijms16010805
Gasparotto Junior A, Gasparotto FM, Lourenço EL, Crestani S, Stefanello ME, Salvador MJ, da Silva-Santos JE, Marques MC, Kassuya CA (2011) Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: evidence for the inhibition of angiotensin converting enzyme. J Ethnopharmacol 134(2):363–372. https://doi.org/10.1016/j.jep.2010.12.026
Bazylko A, Parzonko A, Jeż W, Osińska E, Kiss AK (2014) Inhibition of ROS production, photoprotection, and total phenolic, flavonoids and ascorbic acid content of fresh herb juice and extracts from the leaves and flowers of Tropaeolum majus. Ind Crops Prod 55:19–24. https://doi.org/10.1016/j.indcrop.2014.01.056
Barros RGC, Andrade JKS, Pereira UC, de Oliveira CS, Rezende YRRS, Oliveira Matos Silva T, Nogueira JP, Gualberto NC, Caroline Santos Araujo H, Narain N (2020) Phytochemicals screening, antioxidant capacity and chemometric characterization of four edible flowers from Brazil. Food Res Int 130:108899. https://doi.org/10.1016/j.foodres.2019.108899
Kandil MAM, Sabry RM, Ahmed SS (2016) Influence of drying methods on the quality of sage (Salvia officinalis), parsley (Petroselinum crispum) and nasturtium (Tropaeolum majus). Res J Pharm, Biol Chem Sci 7(4):1112–1123
Ares AM, Nozal MJ, Bernal JL, Bernal J (2014) Optimized extraction, separation and quantification of twelve intact glucosinolates in broccoli leaves. Food Chem 152:66–74. https://doi.org/10.1016/j.foodchem.2013.11.125
Kleinwächter M, Schnug E, Selmar D (2008) The glucosinolate – myrosinase system in nasturtium (Tropaeolum majus L.): Variability of biochemical parameters and screening for clones feasible for pharmaceutical utilization. J Agric Food Chem 56(23):11165–11170. https://doi.org/10.1021/jf802053n
Thomas M, Badr A, Desjardins Y, Gosselin A, Angers P (2018) Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem 245:1204–1211. https://doi.org/10.1016/j.foodchem.2017.11.021
Jo JS, Bhandari SR, Kang GH, Lee JG (2016) Comparative analysis of individual glucosinolates, phytochemicals, and antioxidant activities in broccoli breeding lines. Hortic Environ Biotechnol 57(4):392–403. https://doi.org/10.1007/s13580-016-0088-7
Radošević K, Srček VG, Bubalo MC, Brnčić SR, Takács K, Redovniković IR (2017) Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J Food Compos Anal 61:59–66. https://doi.org/10.1016/j.jfca.2017.02.001
Rutnakornpituk B, Boonthip C, Sanguankul W, Sawangsup P, Rutnakornpituk M (2018) Study in total phenolic contents, antioxidant activity and analysis of glucosinolate compounds in cruciferous vegetables. Naresuan Univ J: Sci Technol 26(2):27–37
Taverniers I, De Loose M, Van Bockstaele E (2004) Trends in quality in the analytical laboratory, II: analytical method validation and quality assurance. TrAC-Trends Anal Chem 23(8):535–552. https://doi.org/10.1016/j.trac.2004.04.001
Thompson M, Ellison S, Wood R (2002) Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl Chem 74(5):835–855. https://doi.org/10.1351/pac200274050835
AOAC International (2016). Guidelines for Standard Method Performance Requirements. In G.W. Latimer, Jr. (Ed.), Official Methods of Analysis of AOAC International (20th ed., Appendix F). AOAC International. http://www.eoma.aoac.org/app_f.pdf. Accessed 1 June 2022
Šilarová P, Česlová L, Meloun M (2017) Fast gradient HPLC/MS separation of phenolics in green tea to monitor their degradation. Food Chem 237:471–480. https://doi.org/10.1016/j.foodchem.2017.05.133
Rivero-Pérez MD, Muňiz P, González-Sanjose ML (2007) Antioxidant profile of red wines evaluated by total antioxidant capacity, scavenger activity, and biomarkers of oxidative stress methodologies. J Agric Food Chem 55:5476–5483. https://doi.org/10.1021/jf070306q
Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr Protoc Food Analytical Chem. https://doi.org/10.1002/0471142913.faf0102s00
Clifford MN, Kirkpatrick J, Kuhnert N, Roozendaal H, Salgado PR (2008) LC–MSn analysis of the cis isomers of chlorogenic acids. Food Chem 106:379–385. https://doi.org/10.1016/j.foodchem.2007.05.081
Ncube EN, Mhlongo MI, Piater LA, Steenkamp PA, Dubery IA, Madala NE (2014) Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacum tissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem Central J. https://doi.org/10.1186/s13065-014-0066-z
Platzer M, Kiese S, Herfellner T, Schweiggert-Weisz U, Miesbauer O, Eisner P (2021) Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 26(5):1244. https://doi.org/10.3390/molecules26051244
Fukalova Fukalova T, García Martínez MD, Raigón MD (2021) Five undervalued edible species inherent to autumn-winter season: nutritional composition, bioactive constituents and volatiles profile. PeerJ 9:e12488. https://doi.org/10.7717/peerj.12488
Rop O, Mlcek J, Jurikova T, Neugebauerova J, Vabkova J (2012) Edible flowers—a new promising source of mineral elements in human nutrition. Molecules 17(6):6672–6683. https://doi.org/10.3390/molecules17066672
Acknowledgements
The project SGS_2021_001 of University of Pardubice is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
LČ: conceptualization, methodology, formal analysis, writing–review and editing, supervision. JK: data curation, writing–original draft. TŠ: investigation, data curation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Compliance with ethics requirement
This study does not involve any human or animal testing.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Česlová, L., Klikarová, J. & Šalomounová, T. The content and profile of biologically active compounds present in individual parts of nasturtium (Tropaeolum majus L.): comprehensive study. Eur Food Res Technol 249, 413–428 (2023). https://doi.org/10.1007/s00217-022-04126-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04126-4