Abstract
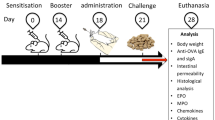
The difference in the allergenicity of ovalbumin between chicken egg and duck egg warrants further investigation. Here, we established an allergic animal model to explore the sensitization difference between chicken OVA (C-OVA) and duck OVA (D-OVA), as well as cross-reactions induced by consumption of D-OVA in C-OVA hypersensitive mice. In our study, both C-OVA and D-OVA induced Th2 cell immune responses, which increased the expression of IL-4, IL-5 and IL-6. the impairment of intestinal barrier, and the decreased expression of Ocln, Claudin-1, ZO-1, ZO-2 and ZO-3. Meanwhile, we also found the cytokine of Th1 cells were increased, including IFN-γ and IL-12, which may be induced through TLR4-NF-κB signaling pathway. Furthermore, the alpha-diversity of intestinal community increased both in C-OVA and D-OVA allergy mice, including richness of Lachnospiraceae, Oscillospiraceae, Ruminococcaceae and Butyricicoccaceae. However, C-OVA induced the strongest allergic reaction, while D-OVA induced a mild Th2 cell immune response and maintained the integrity of the intestinal barrier. D-OVA induced cross-reactions and triggered a milder Th2 cellular immune response, less impairment of intestinal barrier. The results showed that D-OVA caused lower allergic symptoms than C-OVA, and C-OVA allergic mice induced by D-OVA had milder allergic cross-reactions.







Similar content being viewed by others
References
Celebioglu E, Akarsu A, Sahiner UM (2021) IgE-mediated food allergy throughout life. Turk J Med Sci 51(1):49–60. https://doi.org/10.3906/sag-2006-95
Lee AJ, Thalayasingam M, Lee BW (2013) Food allergy in Asia: how does it compare? Asia Pac Allergy 3(1):3–14. https://doi.org/10.5415/apallergy.2013.3.1.3
Ebisawa M, Ito K, Fujisawa T, Comm Japanese Pediat Guideline F, Japanese Soc Pediat Allergy C, Japanese Soc A (2020) Japanese guidelines for food allergy 2020. Allergol Int 69(3):370–386. https://doi.org/10.1016/j.alit.2020.03.004
Jiang N, Yin J, Wen L, Li H (2016) Characteristics of anaphylaxis in 907 Chinese patients referred to a tertiary allergy center: a retrospective study of 1,952 episodes. Allergy Asthma Immunol Res 8(4):353–361. https://doi.org/10.4168/aair.2016.8.4.353
Lee S-Y, Ahn K, Kim J, Jang GC, Min TK, Yang H-J, Pyun BY, Kwon J-W, Sohn MH, Kim KW, Kim K-E, Yu J, Hong S-J, Kwon JH, Kim S-W, Song TW, Kim WK, Kim HY, Jeon YH, Lee YJ, Lee HR, Kim H-Y, Ahn Y, Yum HY, Suh DI, Kim HH, Kim J-T, Kim JH, Park YM, Lee S, Korean Acad Pediat Allergy R (2016) A multicenter retrospective case study of anaphylaxis triggers by age in Korean children. Allergy, Asthma Immunol Res 8(6):535–540. https://doi.org/10.4168/aair.2016.8.6.535
Liew WK, Chiang WC, Goh AE, Lim HH, Chay OM, Chang S, Tan JH, Shih EC, Kidon M (2013) Paediatric anaphylaxis in a Singaporean children cohort: changing food allergy triggers over time. Asia Pac Allergy 3(1):29–34. https://doi.org/10.5415/apallergy.2013.3.1.29
Chen J, Liao Y, Zhang H-z, Zhao H, Chen J, Li H-q (2012) Prevalence of food allergy in children under 2 years of age in three cities in China. Zhonghua er ke za zhi Chin J Pediatrics 50(1):5–9
Ma XJ, Liang R, Xing QL, Lozano-Ojalvo D (2020) Can food processing produce hypoallergenic egg? J Food Sci 85(9):2635–2644. https://doi.org/10.1111/1750-3841.15360
Zhao LP, Wang YP (2015) Market research and forecast of China laying duck industry in 2015. China Poul 37(06):33−36
Moghtaderi M, Nabavizadeh SH, Teshnizi SH (2020) The frequency of cross-reactivity with various avian eggs among children with hen’s egg allergy using skin prick test results: fewer sensitizations with pigeon and goose egg. Allergol Immunopathol 48(3):265–269. https://doi.org/10.1016/j.aller.2019.10.002
De Silva C, Dhanapala P, Doran T, Tang MLK, Suphioglu C (2016) Molecular and immunological analysis of hen’s egg yolk allergens with a focus on YGP42 (Gal d 6). Mol Immunol. https://doi.org/10.1016/j.molimm.2016.02.005
Tan JW, Joshi P (2014) Egg allergy: an update. J Paediatr Child Health 50(1):11–15. https://doi.org/10.1111/jpc.12408
Huntington JA, Stein PE (2001) Structure and properties of ovalbumin. J Chromatogr B 756(1–2):189–198. https://doi.org/10.1016/s0378-4347(01)00108-6
Mine Y, Zhang JW (2002) Comparative studies on antigenicity and allergenicity of native and denatured egg white proteins. J Agric Food Chem 50(9):2679–2683. https://doi.org/10.1021/jf0112264
Mine Y, Yang M (2007) Epitope characterization of ovalbumin in BALB/c mice using different entry routes. Biochim Et Biophys Acta-Proteins Proteom 1774(2):200–212. https://doi.org/10.1016/j.bbapap.2006.12.003
Meng YQ, Qiu N, Geng F, Huo YQ, Sun HH, Keast R (2019) Identification of the duck egg white N-glycoproteome and insight into the course of biological evolution. J Agric Food Chem 67(35):9950–9957. https://doi.org/10.1021/acs.jafc.9b03059
Geng F, Wang J, Liu D, Jin Y, Ma M (2017) Identification of N-Glycosites in chicken egg white proteins using an omics strategy. J Agric Food Chem 65(26):5357–5364. https://doi.org/10.1021/acs.jafc.7b01706
Geng F, Xie YX, Wang JQ, Li SG, Jin YG, Ma MH (2019) Large-scale purification of ovalbumin using polyethylene glycol precipitation and isoelectric precipitation. Poult Sci 98(3):1545–1550. https://doi.org/10.3382/ps/pey402
Pablos-Tanarro A, Lozano-Ojalvo D, Molina E, Lopez-Fandino R (2018) Assessment of the allergenic potential of the main egg white proteins in BALB/c mice. J Agric Food Chem 66(11):2970–2976. https://doi.org/10.1021/acs.jafc.8b00402
Marsteller NL, Goodman RE, Andoh-Kumi K, Luan FL, Bogh KL, Baumert J (2020) Evaluating the potential allergenicity of dietary proteins using model strong to non-allergenic proteins in germ-free mice. Food Chem Toxicol. https://doi.org/10.1016/j.fct.2020.111398
Zhang Z, Xiao H, Zhang X, Zhou P (2019) Insight into the effects of deglycosylation and glycation of shrimp tropomyosin on in vivo allergenicity and mast cell function. Food Funct 10(No. 7):3934–3941. https://doi.org/10.1039/c9fo00699k
Tordesillas L, Berin MC, Sampson HA (2017) Immunology of Food Allergy. Immunity 47(1):32–50. https://doi.org/10.1016/j.immuni.2017.07.004
Xu S, Liu Q, Xiao A, Maleki S, Alcocer M, Gao Y, Cao M, Liu G (2017) Eucheuma cottonii sulfated oligosaccharides decrease food allergic responses in animal models by up-regulating regulatory T (Treg) Cells. J Agric Food Chem 65(No. 15):3212–3222. https://doi.org/10.1021/acs.jafc.7b00389
Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Wegrzyn A (2011) Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol 127(4):990-U244. https://doi.org/10.1016/j.jaci.2011.01.057
Pablos-Tanarro A, Lozano-Ojalvo D, Martinez-Blanco M, Lopez-Fandino R, Molina E (2017) Sensitizing and eliciting capacity of egg white proteins in BALB/c mice as affected by processing. J Agric Food Chem 65(22):4500–4508. https://doi.org/10.1021/acs.jafc.7b00953
Lee D, Kim HS, Shin E, Do SG, Lee CK, Kim YM, Lee MB, Min KY, Koo J, Kim SJ, Nam ST, Kim HW, Park YH, Choi WS (2018) Polysaccharide isolated from Aloe vera gel suppresses ovalbumin-induced food allergy through inhibition of Th2 immunity in mice. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2018.02.061
Elenkov IJ, Chrousos GP (1999) Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab 10(9):359–368. https://doi.org/10.1016/s1043-2760(99)00188-5
Perrier C, Thierry AC, Mercenier A, Corthesy B (2010) Allergen-specific antibody and cytokine responses, mast cell reactivity and intestinal permeability upon oral challenge of sensitized and tolerized mice. Clin Exp Allergy 40(1):153–162. https://doi.org/10.1111/j.1365-2222.2009.03329.x
Ike K, Kameyama N, Ito A, Imai S (2012) Induction of a T-Helper 1 (Th1) immune response in mice by an extract from the Pleurotus eryngii (Eringi) mushroom. J Med Food 15(12):1124–1128. https://doi.org/10.1089/jmf.2012.2239
Holen E, Bolann B, Elsayed S (2001) Novel B and T cell epitopes of chicken ovomucoid (Gal d 1) induce T cell secretion of IL-6, IL-13, and IFN-gamma. Clin Exp Allergy 31(6):952–964. https://doi.org/10.1046/j.1365-2222.2001.01102.x
Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A (2009) MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2-and TLR4-mediated NF-kappa B proinflammatory responses. J Biol Chem 284(36):24192–24203. https://doi.org/10.1074/jbc.M109.023044
Akira S (2013) Pathogen recognition receptors and innate immunity. Xenotransplantation 20(5):351–351
Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, Young HA (1997) Interaction of NF-κB and NFAT with the Interferon-γ Promoter. J Biol Chem 272(48):30412–30420. https://doi.org/10.1074/jbc.272.48.30412
Ali A, Tan HY, Kaiko GE (2020) Role of the intestinal epithelium and its interaction with the microbiota in food allergy. Front Immunol. https://doi.org/10.3389/fimmu.2020.604054
Groschwitz KR, Hogan SP (2009) Intestinal barrier function: Molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124(1):3–20. https://doi.org/10.1016/j.jaci.2009.05.038
Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR (2002) A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123(1):163–172. https://doi.org/10.1053/gast.2002.34235
Tavalali S, Schmitz H, Fromm M, Schulzke JD, Mankertz JT (2000) Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. Gastroenterology 118(4):A602–A602. https://doi.org/10.1016/s0016-5085(00)84547-3
Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE (2003) Tight junction proteins. Prog Biophys Mol Biol 81(1):1–44. https://doi.org/10.1016/s0079-6107(02)00037-8
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2(4):285–293. https://doi.org/10.1038/35067088
Monaco A, Ovryn B, Axis J, Amsler K (2021) The epithelial cell leak pathway. Int J Mol Sci. https://doi.org/10.3390/ijms22147677
Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273(45):29745–29753. https://doi.org/10.1074/jbc.273.45.29745
Itoh M, Nagafuchi A, Moroi S, Tsukita S (1997) Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to or catenin and actin filaments. J Cell Biol 138(1):181–192. https://doi.org/10.1083/jcb.138.1.181
Jackson MA, Verdi S, Maxan ME, Shin CM, Zierer J, Bowyer RCE, Martin T, Williams FMK, Menni C, Bell JT, Spector TD, Steves CJ (2018) Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. https://doi.org/10.1038/s41467-018-05184-7
Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, Yuan L, Wang Y, Sun J, Li L, Xiang C (2014) Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol 80(8):2546–2554. https://doi.org/10.1128/aem.00003-14
Fazlollahi M, Chun Y, Grishin A, Wood RA, Burks AW, Dawson P, Jones SM, Leung DYM, Sampson HA, Sicherer SH, Bunyavanich S (2018) Early-life gut microbiome and egg allergy. Allergy 73(7):1515–1524. https://doi.org/10.1111/all.13389
Chua HH, Chou HC, Tung YL, Chiang BL, Liao CC, Liu HH, Ni YH (2018) Intestinal dysbiosis featuring abundance of & ITRuminococcus gnavus & ITAssociates with allergic diseases in infants. Gastroenterology 154(1):154–167. https://doi.org/10.1053/j.gastro.2017.09.006
Lee KH, Guo J, Song Y, Ariff A, O’Sullivan M, Hales B, Mullins BJ, Zhang GC (2021) Dysfunctional gut microbiome networks in childhood IgE-mediated food allergy. Int J Mol Sci. https://doi.org/10.3390/ijms22042079
Bunyavanich S, Berin MC (2019) Food allergy and the microbiome: current understandings and future directions. J Allergy Clin Immunol 144(6):1468–1477. https://doi.org/10.1016/j.jaci.2019.10.019
Mauras A, Wopereis H, Yeop I, Esber N, Delannoy J, Labellie C, Reygner J, Kapel N, Slump R, van Eijndthoven T, Rutten L, Knol J, Garssen J, Harthoorn LF, Butel MJ, Bajaj-Elliott M, Hartog A, Waligora-Dupriet AJ (2019) Gut microbiota from infant with cow’s milk allergy promotes clinical and immune features of atopy in a murine model. Allergy 74(9):1790–1793. https://doi.org/10.1111/all.13787
Canani RB, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, Calignano A, Khan AA, Gilbert JA, Nagler CR (2016) Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J 10(3):742–750. https://doi.org/10.1038/ismej.2015.151
Kameyama K, Itoii K (2014) Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ 29(4):427–430. https://doi.org/10.1264/jsme2.ME14054
Nakanishi Y, Sato T, Ohteki T (2015) Commensal gram-positive bacteria initiates colitis by inducing monocyte/macrophage mobilization. Mucosal Immunol 8(1):152–160. https://doi.org/10.1038/mi.2014.53
Leonard MM, Valitutti F, Karathia H, Pujolassos M, Kenyon V, Fanelli B, Troisi J, Subramanian P, Camhi S, Colucci A, Serena G, Cucchiara S, Trovato CM, Malamisura B, Francavilla R, Elli L, Hasan NA, Zomorrodi AR, Colwell R, Fasano A (2021) Microbiome signatures of progression toward celiac disease onset in at-risk children in a longitudinal cohort. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2020322118
Alam MS, Gangiredla J, Hasan NA, Barnaba T, Tartera C (2021) Aging-induced dysbiosis of gut microbiota as a risk factor for increased listeria monocytogenes infection. Front Immunol. https://doi.org/10.3389/fimmu.2021.672353
Ott SJ, Kuhbacher T, Musfeldt M, Rosenstiel P, Hellmig S, Rehman A, Drews O, Weichert W, Timmis KN, Schreiber S (2008) Fungi and inflammatory bowel diseases: alterations of composition and diversity. Scand J Gastroenterol 43(7):831–841. https://doi.org/10.1080/00365520801935434
Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD (2013) Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol 131(2):346. https://doi.org/10.1016/j.jaci.2012.11.013
Huang YJ, Nelson CE, Brodie EL, DeSantis TZ, Baek MS, Liu JN, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, Sutherland ER, King TS, Icitovic N, Martin RJ, Calhoun WJ, Castro M, Denlinger LC, DiMango E, Kraft M, Peters SP, Wasserman SI, Wechsler ME, Boushey HA, Lynch SV, Natl Heart L, Blood Inst A (2011) Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol 127(2):372-U689. https://doi.org/10.1016/j.jaci.2010.10.048
Bunyavanich S, Schadt EE (2015) Systems biology of asthma and allergic diseases: a multiscale approach. J Allergy Clin Immunol 135(1):31–42. https://doi.org/10.1016/j.jaci.2014.10.015
Huang YJ, Marsland BJ, Bunyavanich S, O’Mahony L, Leung DYM, Muraro A, Fleisher TA (2017) The microbiome in allergic disease: current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol 139(4):1099–1110. https://doi.org/10.1016/j.jaci.2017.02.007
Park HY, Yoon TJ, Kim HH, Han YS, Choi HD (2017) Changes in the antigenicity and allergenicity of ovalbumin in chicken egg white by N-acetylglucosaminidase. Food Chem 217:342–345. https://doi.org/10.1016/j.foodchem.2016.08.112
Liu TG, Navarro S, Lopata AL (2016) Current advances of murine models for food allergy. Mol Immunol. https://doi.org/10.1016/j.molimm.2015.11.011
Graham MT, Nadeau KC (2014) Lessons learned from mice and man: mimicking human allergy through mouse models. Clin Immunol 155(1):1–16. https://doi.org/10.1016/j.clim.2014.08.002
Gonipeta B, Kim E, Gangur V (2015) Mouse models of food allergy: how well do they simulate the human disorder? Crit Rev Food Sci Nutr 55(3):437–452. https://doi.org/10.1080/10408398.2012.657807
Acknowledgements
This work was supported by the State Key Research and Development Plan (2019YFC1605002), National Natural Science Foundation of China (31801463), Innovation and Exploration Project of State Key Laboratory of Food Science and Technology (SKLF-ZZA-202104).
Author information
Authors and Affiliations
Contributions
1. PZ and LZ conceived the concept of the study and provided supervision. 2. RZ and LZ wrote the manuscript. 3. RZ carried out all experiments, data analysis. 4. KZ participate in the concept of the study and carried out part of experiments. In summary: RZ: methodology, software, formal analysis, investigation, data curation, writing—original draft. LZ: conceptualization, methodology, validation, writing—review & editing, Supervision; funding acquisition. KZ: conceptualization, methodology, data curation. PZ: conceptualization, supervision, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
The mice were obtained from Charles River (Suzhou, Jiangsu, China) with license number JN.No20201130b0800120 [351], and all animal experiments complied with the ARRIVE guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, R., Zhang, L., Zhang, K. et al. Difference of egg ovalbumin sensitization between egg and duck eggs in BALB/c mice. Eur Food Res Technol 248, 1035–1048 (2022). https://doi.org/10.1007/s00217-021-03943-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03943-3