Abstract
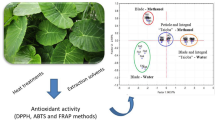
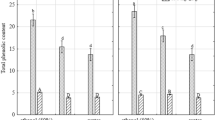
This study aimed to investigate the phenolic composition and antioxidant capacity of Inula viscosa L. aerial parts as influenced by their geographic origin and the type of extractant used. We established the extraction yield and phenolic composition of I. viscosa plants supplied from Mediterranean, Aegean, and Black Sea regions and evaluated their antioxidant capacity. Hierarchical clustering agglomerative (CA) and principal components analysis (PCA) was performed for further evaluation of similarities and differences among the I. viscosa extracts. Based on PCA and CA, the plants were specified in three distinct groups; one group presented higher bioactive composition and more potent antioxidative properties. The extractant type was one of the parameters affecting the clustering of the plants on the PCA biplot and CA dendrogram. Amongst the screened plants, Plant 1 was discriminated by its higher extraction efficiency, bioactive compounds, and antioxidant capacity compared to other plants. Ethanol was the most effective extractant studied when compared with ethyl acetate and hexane in terms of extraction yield, phenolic composition, and antioxidant capacity. Due to our findings, the phenolic composition was successfully used as a biochemical indicator to specify natural I. viscosa plants. The results highlighted that I. viscosa plant could be an excellent natural source of antioxidants to be evaluated in food and pharmaceutical industries.









Similar content being viewed by others
References
Anokwuru C, Sigidi M, Boukandou M, Tshisikhawe P, Traore A, Potgieter N (2018) Molecules 23:1303
Yang R, Guan Y, Wang WX, Chen HJ, He ZC, Jia AQ (2018) PLoS ONE 13:e0195508
Ulewicz-Magulska B, Wesolowski M (2019) Plant Foods Hum Nut 74:61–67
Kumar S, Yadav A, Yadav M, Yadav JP (2017) BMC Res Notes 10:60
Rhimi W, Ben Salem I, Iatta R, Chaabane H, Saidi M, Boulila A, Cafarchia C (2018) Ind Crops Prod 113:196–201
Gokbulut A, Ozhan O, Satilmis B, Batcioglu K, Gunal S, Sarer E (2013) Nat Prod Commun 8:475–478
Brahmi-Chendouh N, Piccolella S, Crescente G, Pacifico F, Boulekbache L, Hamri-Zeghichi S, Akkal S, Madani K, Pacifico S (2019) J Food Drug Anal 27:692–702
Mahmoudi H, Hosni K, Zaouali W, Amri I, Zargouni H, Ben Hamida N, Kaddour R, Hamrouni L, Ben Nasri M, Ouerghi Z (2016) J Food S 36:77–88
Talib WH, Zarga MH, Mahasneh AM (2012) Molecules 17:3291–3303
Seca AML, Grigore A, Pinto DCGA, Silva AMS (2014) J Ethnopharmacol 154:286–310
Scribble Maps. https://www.scribblemaps.com. Accessed on 20 May 2021.
Ghorbanzadeh R, Rezaei K (2017) J Am Oil Chem Soc 94:1491–1501
Maisuthisakul P, Suttajit M, Pongsawatmanit R (2007) Food chem 100:1409–1418
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) J Food Drug Anal 10:3
Price ML, Van Scoyoc S, Butler LG (1978) J Agric Food Chem 26:1214–1218
Chan EWC, Lim YY, Ling SK, Tan SP, Lim K, Khoo MG (2009) LWT Food Sci Technol 42:1026–1030
Prasad NK, Yang B, Zhao MM, Wang BS, Chen F, Jiang YM (2009) Int J Food Sci Technol 44:960–966
Sultana B, Anwar F, Ashraf M (2009) Molecules 14:2167–2180
Hsu B, Coupar IM, Ng K (2006) Food Chem 98:317–328
Chahmi N, Anissi J, Jennan S, Farah A, Sendide K, El Hassouni M (2015) Asian Pac J Trop Biomed 5:228–233
Salim H, Rimawi WH, Mjahed A (2017) J Chem and Biochem 5:12
Iloki-Assanga SB, Lewis-Luján LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL, Haines DD (2015) BMC Res Notes 8:1–14
Li H, Zhang D, Tan LH, Yu B, Zhao SP, Cao WG (2017) Afr J Bot 109:1–8
Sriti Eljazi J, Selmi S, Zarroug Y, Wesleti I, Aouini B, Jallouli S, Limam F (2018) Int J Food Prop 21:2309–2319
Kumar S, Sandhir R, Ojha S (2014) BMC Res Notes 7:560
Piluzza G, Bullitta S (2011) Pharma Biol 49:240–247
Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N (2019) Plants (Basel) 8:96
Wianowska D, Gil M (2019) Phytochem Rev 18:273–302
Rotta EM, Haminiuk CWI, Maldaner L, Visentainer JV (2017) Int J Food Sci Technol 52:954–963
Kainama H, Fatmawati S, Santoso M, Papilaya PM, Ersam T (2020) Pharm Chem J 53:1151–1157
Koleckar V, Kubikova K, Rehakova Z, Kuca K, Jun D, Jahodar L, Opletal L (2008) Mini Rev Med Chem 8:436–447
Danino O, Gottlieb HE, Grossman S, Bergman M (2009) Food Res Inter 42:1273–1280
Dolkar P, Dolkar D, Angmo S, Kumar B, Stobdan T (2017) J Ber Res 7:109–116
Kabtni S, Sdouga D, Bettaib Rebey I, Save M, Trifi-Farah N, Fauconnier ML, Marghali S (2020) Sci Rep 10:8293
Zargoosh Z, Ghavam M, Bacchetta G, Tavili A (2019) Sci Rep 9:16021
Verma N, Shukla S (2015) J App Res Med Arom Plants 2:105–113
Acknowledgements
This study was supported financially by the Scientific Research Projects Governing Unit of the University of Gaziantep is [Grant Number MF.YLT.20.01] gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was supported financially by the Scientific Research Projects Governing Unit of the University of Gaziantep, Turkey.
Compliance with ethics requirements
The article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keskin Çavdar, H., Yıldırım, Z.İ. & Fadıloğlu, S. Evaluation of the effect of geographical origin and extraction solvents on bioactive and antioxidative properties of Inula viscosa L. grown in Turkey by chemometric approach. Eur Food Res Technol 248, 253–261 (2022). https://doi.org/10.1007/s00217-021-03877-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03877-w