Abstract
This study investigated the efficacy of three commercially relevant packaging methods (vacuum with water glazing VAC-G; vacuum with seawater VAC-S; shatter-layer packaging SL) to improve frozen storage stability of mechanically filleted Atlantic mackerel at − 25 °C, in comparison to water glazing alone (GL) and storage as whole unglazed, block frozen fish. Besides proximate composition and pH of raw material, quality changes were analysed by free fatty acid content (FFA), water holding capacity (WHC), cooking yield, lipid oxidation (lipid hydroperoxides, PV; non-protein bound thiobarbituric acid reactive substances, TBARS) and sensory profiles of cooked samples after 3.5, 8, 10 and 12 months of frozen storage. Vacuum-packaging was effective in mitigating the PV and TBARS as well as rancid odour and flavour. The inclusion of seawater in VAC-S altered the sensory textural attributes of the mackerel fillet to be more juicy, tender and soft and increased the attribute of salty flavour in the sample. SL delayed rancid odour and flavour by 2 months compared to GL. Processing of mackerel under industrial conditions, including filleting, handling, double-freezing and glazing accelerated the formation of FFA as well as losses of WHC and cooking yield in the fillet regardless the packaging methods.
Similar content being viewed by others
Introduction
Mackerel is a good source of protein, fat soluble vitamins and minerals as well as long chain n-3 polyunsaturated fatty acids (LC n-3 PUFA), including EPA and DHA. Currently, few Atlantic mackerel is being processed (filleted) prior to export from the northern European countries, and frozen blocks of whole or headed and gutted mackerel are thawed and processed into fillets at international processors. A series of national initiatives are being undertaken in the Nordic countries to process mackerel into high valued frozen fillets, thus to achieve more sustainable biomass utilisation and value creation through the utilisation of rest raw materials into e.g. fish meal and fish oil. Mackerel muscle is highly susceptible to lipid oxidation during frozen storage because of its low post-mortem pH, high PUFA content and abundance of pro-oxidants [e.g. haemoglobin (Hb) and myoglobin (Mb), metallic ions such as iron and copper] [10, 25]. Further, mechanical damage and increased exposure to oxygen upon filleting make PUFA-rich mackerel fillets highly susceptible to oxidative degradation [2, 4]. The rate and extent of quality loss of mackerel muscle during frozen storage may be influenced by storage temperature [26], season, feeding state [4, 29], total fat content, lipid composition [18], and concentrations of endogenous anti- and pro-oxidants [23, 25].
Additions of both synthetic and natural antioxidants have been extensively studied using mackerel mince [32]. Secondary processing of mackerel species, e.g. sou-vide cooking, smoking and use of other advanced technologies such as ultrasound and high-pressure treatment are well dealt with in the recent literature [7, 9, 15, 22]. However, other means of shelf life extension, which are also suitable for intact fillets, remain under-researched despite commercial relevance and importance for product quality and acceptance. For example, glazing of mackerel fillets with water is used commercially, e.g. when fillets are exported from a processing country to a consumption market, while its effects and potential application have not been fully investigated within research [24]. Vacuum packaging have been evaluated to limit lipid oxidation and enhance storage stability of mackerel fillets [31, 37] but not tested for mackerel mechanically filleted under industrial settings. Moreover, bulk vacuum-packaging with seawater is widely used in the Norwegian pelagic industry to store mackerel and herring fillets intended for secondary processing such as canning. Shatter layer packaging is commonly used onboard Icelandic white fish freezing trawlers with polyethylene sheets wrapping fillets to prevent them from sticking together. Despite these applications within industry, as well as the scattered research studies, no one has, to the best of our knowledge, systematically compared the abovementioned packaging methods for mackerel fillets processed under industrial conditions. The objective of this study was to investigate the efficacy of three packaging methods (vacuum with water glazing VAC-G; vacuum with seawater VAC-S; shatter-layer packaging SL) for improving frozen storage stability of mechanically filleted Atlantic mackerel fillets at − 25 °C, in comparison to water glazing alone (GL) and storage as whole non-glazed block frozen fish (WF). In addition to proximate composition and pH of the raw material, quality changes were analysed by free fatty acid content (FFA), water holding capacity (WHC), cooking yield, lipid oxidation (PV and TBARS) and sensory profiles of cooked samples at 3.5, 8, 10 and 12 months of storage.
Materials and methods
Raw material and processing
Atlantic mackerel caught in August 2016 in the waters southeast of Iceland (northeast Atlantic Ocean—FAO no 27) by amid-water pelagic trawl of a modern purse seiner (Börkur NK 122) was kept in a tank with refrigerated seawater (− 1.5 °C) for 2 days. Upon landing and sorting, whole fish was superchilled in 15% brine at − 10 °C for 15–30 min or until the core temperature of the fish was in the range of − 1.0 to − 1.5 °C. The ratio of fish and brine was 1:2. The superchilled fish was mechanically filleted (MK11-M120, Arenco VMK, Sweden). Immediately after filleting, individual fillets were placed on the conveyer belt and transported to an IQF freezer (Skaginn, Akranes, Iceland) at − 42 °C (air speed at 15–20 m/s). Whole mackerel were frozen using an automatic plate freezer (Skaginn, Akranes, Iceland) at − 45 °C into typical industrial frozen blocks (16 kg). The frozen blocks were thereafter stored at − 25 °C.
Glazing and packaging of mackerel fillets
Frozen fillets were divided into four groups corresponding to water glazing (GL), vacuum-packaging with water glazing (VAC-G), vacuum-packaging with seawater (VAC-S) and shatter layer packaging (SL), respectively. GL and VAC-G fillets were individually glazed by dipping in a container with iced water for 3–4 s. Ice was added to the water to ensure the low temperature. Immediately after glazing, GL samples were randomly packed in LDPE plastic bags (Kivo, Volendam, Netherland) and corrugated cardboard boxes (390 × 190 × 170 mm, Smurfit Kappa Norpapp AS, Dublin, Ireland). Six glazed fillets were vacuum packaged (Supervac RP 00791, Wien) in each 0.130-mm thick polyamide/polyethylene (PA/PE) bag (300 × 550 mm) for VAC-G. Unglazed SL fillets were arranged systematically on a LDPE plastic film and placed in LDPE plastic bags and corrugated cardboard boxes. For VAC-S, seawater at 0 °C was added into a 75-μm thick LDPE bag with unglazed fillets to obtain a seawater:fish ratio of 1:4. The bags were vacuum-packed at 92% (Supervac RP 00791, Wien) and frozen using an automatic box freezer at − 25 °C (Skaginn, Akranes, Iceland). Following packaging, all samples were loaded on pallets and transported frozen (− 20 °C) to the laboratory for subsequent frozen storage at − 25 °C.
The physicochemical and sensory analyses were performed after 3.5, 8, 10 and 12 months of frozen storage. At each sampling point, one frozen block of WF were thawed at room temperature (21 °C) for 17 h and filleted manually prior to analysis. Six fillets of GL, VAC-G, VAC-S and SL samples were thawed at 0 °C for 12 h in an open carton box covered with a plastic film. For the PV and TBARS analyses, frozen WF and fillet samples were thawed in a sealed plastic bag under cold running water. For each physicochemical analysis, 3 thawed fillets (i.e. N = 3) were pooled into a mince using a food processor (Braun Electronic, type 4262, Kronberg, Germany) and 2–3 samples from this mince were subjected to the respective analysis (thus, a = 2–3). The mince was stored at − 80 °C until the physicochemical analyses. In the sensory analyses, the belly and the tail parts of the fillets were trimmed off, and the centre section was used as described below in “Cooking yield”.
Water, lipid, protein and salt content
Water content was determined by the difference in weight of homogenized muscle samples before and after drying for 4 h at 102–104 °C (ISO, 1999). Results were calculated as g water per 100 g muscle. Total lipids (TL) of the muscle samples were extracted according to the method of Bligh and Dyer [6]. The lipid content was determined gravimetrically, and the results were expressed as g lipid per 100 g of muscle. The total protein content of the fish muscle was estimated by the Kjeldahl method (ISO, 1997) with the aid of a Digestion System 40 (Tecator AB, Hoganas, Sweden) and calculated using total nitrogen (N) × 6.25. Salt content was determined by the method of Volhard according to the AOAC Official Methods of Analysis (2000).
pH
The pH of fish muscle was determined using a pH meter with a glass electrode (Knick, Berlin, Germany) by inserting the electrode directly into the mince (Braun Electronic, type 4262, Kronberg, Germany).
Fatty acid profile
The fatty acid profile of the samples was measured on the TL extract by gas chromatography of fatty acid methyl esters (FAMEs) (Varian 3900 GC, Varian, Inc., Walnut Creek, CA, USA), according to the AOCS method (AOCS 1998). The Varian 3900 GC equipped with a fused silica capillary column (HP-88, 100 × 0.25 μm film), split injector, and flame ionization detector with a Galaxie Chromatography Data System (Version 1.9.3.2 software, Varian Inc., Walnut Creek, CA, USA) was used. The temperature in the oven was held at 100 °C for 4 min and increased to 240 °C at a rate of 3 °C/min for 15 min. The temperature of the injector and detector was 225 °C and 285 °C, respectively. Helium used as a carrier gas had a column flow of 0.8 mL/min, and a split ratio 200:1. The program was based on the AOAC-996.06 (2001) method.
Free fatty acids content
The free fatty acid (FFA) content was determined as described by Bernárdez et al. [5] with a modification using the method of Lowry and Tinsley [17]. The absorbance of the solution was read at 710 nm (UV-1800 spectrophotometer, Shimadzu, Japan) and the amount of free fatty acids was determined from a standard curve prepared from oleic acid in a concentration range of 2–22 μmol. Results was expressed as grams FFA per 100 g of total lipids.
Water holding capacity
Water holding capacity (WHC) of the samples was determined by a centrifugation method [11]. The WHC was expressed as the percentage of retained weight after centrifugation (1350 rpm, 5 min) at 4 °C, divided by the initial sample weight and multiplied by 100.
Cooking yield
Trimmed fillets as described in “Sensory analysis” were weighed and steam-cooked at 95–100 °C for 8 min in a Convostar oven (Convotherm, Elektrogerate GmbH, Eglfing, Germany) before they were drained for 15 min and weighed again. Cook loss was calculated as the percentage of the weight loss to the initial weight.
Lipid oxidation
Lipid hydroperoxides (PV) and thiobarbituric acid reactive substances (TBARS) were determined in the polar and the non-polar phase, respectively, after a chloroform:methanol extraction. Eight g sample and 80 mL ice-cold chloroform:methanol (2:1; 0.05% BHT) was homogenized (Ultra Turrax T18 basic, IKA Works, Wilmington, NC) for 60 s at 14 000 rpm, followed by the addition of 25 mL ice-cold 0.5% NaCl, vortexing for 30 s, and centrifugation (2000×g, 6 min, 4 °C). Lipid hydroperoxides were determined in the chloroform phase with the ferrithiocyanate method described by Undeland et al. [38]. Quantification was determined using a standard curve prepared from cumene hydroperoxide. Results were expressed as mmol lipid hydroperoxides/kg muscle (a = 3). 1 mmol lipid hydroperoxides/kg muscle is equivalent to 2 meq O2/kg muscle. Non-protein bound TBARS were determined in the methanol–water phase according to the method by [30]. Propyl gallate and EDTA was added to the TCA-TBA solution at a concentration of 0.1% (w/v) to avoid possible oxidation during analysis. The absorbance of the TBA-complex was measured with a spectrophotometer at 532 nm. Quantification of TBARS was made with a standard curve prepared from 1,1,3,3-tetraethoxypropane (TEP). Results were expressed as μmol MDA-equivalents/kg mince (a = 3)”.
Sensory analysis
The descriptive attributes (Table 1) of cooked mackerel fillets were determined using a sensory evaluation (generic descriptive analysis; GDA) [34]. Each fillet was trimmed by removing the belly and the tail and cut in two to three portions. The portions were cooked at 100 °C for 6 min in a pre-heated steam oven (Convotherm Elektrogeräte GmbH, Eglfing, Germany) and served to the trained sensory panel (ISO, 1993) at the serving temperature at 65–75 °C. Each panellist evaluated the samples in duplicates served at a random order. A computerized system (FIZZ, Version 2.0, 1994–2000, Biosystèmes) was used for data recording.
Statistical analysis
Statistical analyses were performed using the SPSS statistics software package version 25 (SPSS Inc., Chicago, IL). Two-way ANOVA was used to examine the significant effects of filleting, packaging method and storage time. One-way ANOVA and Duncan post hoc test were performed to test simple main effects and for the pair-wise comparison, respectively. The significance level cut-off was set at 95% (p < 0.05). Principal component analysis (PCA) was performed using the R program (The R Foundation for Statistical Computing, Vienna). The average scores at 3.5 and 8 months were subjected to the analysis as the sensory evaluation of GL was terminated after 8 months when the average GDA score for the attributes rancid odour and/or rancid flavour reached above 20 and most of the panellists deemed the sample unpalatable. The data were centred, and a PCA bi-plot was obtained to visualize how the packaging methods and the storage time were related to change in the sensory attributes of the samples.
Results and discussion
Raw material characteristics
The average water and lipid content of the mackerel raw material were 59.0 ± 2.2% and 21.1 ± 2.8%, respectively, and neither was significantly affected by storage time nor packaging method. The average values obtained were comparable to earlier reported values for mackerel caught in the Icelandic waters, where significant geographical and annual variations have been reported [26,27, 29]. VAC-S had a significantly higher salt content (0.78 ± 0.13%) than the other fillets (GL, VAC-G, SL) (0.28 ± 0.044%) and WF (0.38 ± 0.084%), possibly due to increased contact with water during the filleting and glazing operations. The muscle pH of the raw material (average 6.3 ± 0.1) was not influenced by storage time or the packaging methods. The obtained pH-value was higher than post-rigor pH reported for mackerel species (~ pH 6) [13, 14]. This could be related to differences in capture/slaughter, post-mortem handling and storage, influencing post mortem depletion of adenosine triphosphate (ATP) and level of glycogen and lactate in mackerel muscle following slaughter [35].
The fatty acid profile of the raw material was characterised by the dominant monounsaturated fatty acids (MUFA, average 40.0 ± 1.02 g/100 g lipids), followed by PUFA (30.9 ± 1.07 g/100 g lipids) and saturated fatty acids (21.4 ± 1.19 g/100 g lipids). Docosahexaenoic acid (C22:6n-3, DHA) (12.3 ± 0.53 g/100 g lipids) and eicosapentaenoic acid (C20:5n-3, EPA) (6.85 ± 0.34 g/100 g lipids) were among the most abundant PUFA in agreement with earlier studies [18, 29]. The total PUFA content in the sample was slightly lower than those previously reported for mackerel caught in the Icelandic waters at the beginning of the heavy feeding period (end of July) (33.9 ± 0.8%) [26], possibly reflecting a natural variation in e.g. feed availability/composition and ocean temperature [12].
Effects of filleting and packaging method on storage stability and quality of mackerel
Lipid oxidation
The PV and TBARS values of the samples are presented in Fig. 1a, b, respectively. The levels of PV and TBARS in WF were comparable to those of GL after 12 months, with a peak observed in both PV and TBARS measurements at 10 months. These results contradict earlier studies demonstrating that storage as whole fish mitigated the increase in PV and TBARS along with the loss of endogenous antioxidant (α-tocopherol) in mackerel at − 20 °C up to 12 months [2, 4]. Vacuum-packaging decreased lipid oxidation in mackerel fillets as indicated by the significantly lower PV and TBARS values in VAC-G and VAC-S compared to GL. The PV and TBARS values of these fillets remained relatively stable. The results are in agreement with others reporting the efficacy of reduced-oxygen packaging for mitigating the lipid oxidation in mackerel [31, 37]. The inclusion of the seawater in vacuum packaging led to a significantly higher TBARS value in VAC-S than VAC-G after 8 months, indicating that the increased salt content turned mackerel muscle more prone to lipid oxidation during storage [1, 27]. NaCl can be pro-oxidative e.g. via activation of myeloperoxidase and by-displacing Fe2+ from protein, liberating more low molecular weight iron [16, 19]. The seawater in the packaging may also have increased the exposure of the mackerel muscle to pro-oxidative compounds in e.g. blood. SL significantly delayed the development of lipid oxidation until 8 months, followed by an increase in both PV and TBARS values to those comparable of GL at 12 months. The results indicated a moderate protective effect of the shatter layer packaging on the lipid oxidation, though not as effective as vacuum packaging.
a Average lipid hydroperoxides (mmol/kg mince) ± standard deviation of samples at 3.5, 8, 10 and 12 months of storage at − 25 °C. The respective samples are represented by a solid line: GL; dotted line: WF; open circle: VAC-G; closed circle: VAC-S; open triangle: SL. Error bars show analytical variation (a = 2–3). b Average thiobarbituric acid reactive substances TBARS (MDA-equivalents µmol/kg muscle) ± standard deviation of samples at 3.5, 8, 10 and 12 months of storage at − 25 °C. The respective samples are represented by a solid line: GL; dotted line: WF; open circle: VAC-G; closed circle: VAC-S; open triangle: SL. Error bars show analytical variation (a = 2–3)
Free fatty acids (FFA)
The average FFA values of samples are presented in Fig. 2. The level of FFA in the muscle increased during storage for all samples. The average FFA concentration at 3.5 months was 1.58 ± 0.30 g/100 g lipid, comparable to those reported for the mackerel caught in the Icelandic waters [28, 29]. The FFA value of WF showed a sharp increase up to 8 months and remained relatively stable until the end of the storage period. The FFA formation in the VAC-G, VAC-S and SL fillets reached a peak at 10 months before it decreased at 12 months. Similar patterns of FFA development were reported during the frozen storage of Atlantic mackerel [4] and horse mackerel [31, 37]. WF was found to be most effective in preventing the formation of FFA compared to GL, though this outcome may have been season dependent [4]. Further, VAC-S significantly mitigated the lipid hydrolysis when compared to GL after 8 months, likely due to the increased salt content in muscle [3]. VAC-G did not reduce the formation of FFA when compared to GL after 8 months. FFA development in SL followed that of GL indicating that the additional layer provided was not sufficient to alleviate the FFA formation in mackerel muscle. Tzikas et al. [37] reported the preservative effect of vacuum packaging on FFA increase in skinned horse mackerel fillets only up to 3 months at − 18 °C. Besides catching season and geographical variation [4, 29], storage time and temperature [27, 28], raw material/sample composition [8, 33], temperature fluctuation [26] has been shown to account for an accelerated increase in FFA during storage. Similarly in this study, processing of mackerel including filleting, handling, double-freezing and glazing may have promoted the subsequent hydrolytic activity of the lipid constituents possibly due to the associated temperature fluctuation, accounting for the higher levels of FFA in the fillets than the whole fish regardless the packaging methods.
Average free fatty acid content (g FFA/100 g lipids) ± standard deviation of samples at 3.5, 8, 10 and 12 months of storage at − 25 °C. The respective samples are represented by a solid line: GL; dotted line: WF; open circle: VAC-G; closed circle: VAC-S; open triangle: SL. Error bars show analytical variation (a = 2–3)
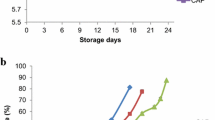
Water holding capacity (WHC) and cooking yield
The average WHC and cooking yield of the samples are presented in Fig. 3a, b, respectively. The WHC of the samples (average 86.8 ± 5.3%) decreased during storage. WF was most protected against loss of WHC as indicated by a higher WHC (78.0 ± 0.75%) at 12 months than those of the fillets (mean range 58.5–68.6%). WF maintained a significantly higher level of the cooking yield at 12 months, while none of the packaging methods mitigated the decrease in WHC or cooking yield when compared to GL. The results indicated that the fillets were subjected to extensive degradation e.g. of the proteins upon filleting, handling and frozen storage. Mitigation of lipid oxidation by e.g. packaging and/or addition of antioxidants may not always result in improved WHC and oxidative stability of muscle proteins in mackerel [20, 21]. Brining at low NaCl-concentrations has been shown to improve the WHC and cooking yield of fish muscle (e.g. with a salt content ~ 1%) due to salt-induced swelling [27, 36], but this positive effect of the salt was not observed for VAC-S, which contained on the average 0.78 ± 0.13% NaCl.
Average water holding capacity (WHC; %) ± standard deviation of samples at 3.5, 8, 10 and 12 months of storage at − 25 °C. The respective samples are represented by a solid line: GL; dotted line: WF; open circle: VAC-G; closed circle: VAC-S; open triangle: SL. Error bars show analytical variation (a = 2–3). b sAverage cooking yield (%) ± standard deviation of samples at 3.5, 8, 10 and 12 months of storage at − 25 °C. The respective samples are represented by a solid line: GL; dotted line: WF; open circle: VAC-G; closed circle: VAC-S; open triangle: SL. Error bars show analytical variation (a = 2–3)
Sensory analysis
Rancidity is the primary cause for sensory rejection of fatty fish such as mackerel, especially in the case of a frozen product. The average scores for rancid odour and flavour in VAC-G and VAC-S were both well below the detection limit (< 20) after 10 months, indicating that vacuum packaging was effective in mitigating rancid odour and flavour during frozen storage. SL delayed the rancidity detection limit by 2 months when compared to GL, providing a sensory shelf life of 10 months. In the PCA bi-plot (Fig. 4), the largest variation in the sensory data (PC1, 81.6%) accounted for the increase in rancid odour and flavour (O.RANCID and F.RANCID), which were found to the right on PC1 near 8 months GL, SL and WF. The fillets at 3.5 months were found on the opposite side of PC1 closely related to sensory attributes such as fresh fish oil odour/flavour (O.OIL and F.OIL), metallic flavour (F.METAL) and sweet characteristic flavour of fresh mackerel (F.SWEET), reflecting loss of these attributes with the increase of rancid odour and flavour over time. Deviation from this was observed in WF and VAC-S. The position of WF at 3.5 months may be explained by a relatively high score of rancid odour (average 14.2 ± 12) and flavour (19.2 ± 14) with a large standard deviation. This may indicate a large batch-to-batch variation in raw material e.g. composition and on-land operation and transport affecting the storage stability of WF during the subsequent storage. VAC-S at 3.5 and 8 months were found to the left along PC1, which indicated that the vacuum-packaging with seawater allowed the samples to retain the positive sensory attributes associated with the samples at 3.5 months (O.OIL, F.OIL, F.METAL and F.SWEET). This did not agree with the development of lipid oxidation measured as TBARS, where a significantly higher value was obtained in VAC-S compared to VAC-G at 8 months. Indeed, the fact that samples were cooked prior to the sensory analyses, but not prior to the TBARS analyses can be an underlying factor changing the profile of secondary oxidation products. VAC-S at 3.5 and 8 months were also closely associated with the textural attributes such as juicy (T.JUICY), tender (T.TENDER), soft (T.SOFT) as well as with the attribute of salty flavour (F.SALTY), indicating that this treatment altered the sensory attributes of the mackerel fillets. PC2 (8%) seemed to account for this variation in the data.
PCA biplot constructed from the average sensory scores of the GL, WF, VAC-G, VAC-S and SL samples at 3.5 and 8 months in light gray and black letters, respectively. The primary x and y axes belong to the PCA score plot of the samples and the secondary axes belong to the loading plot of the sensory attributes (variables), respectively. Refer to Table 1 for the labeled sensory attributes
Conclusions
Vacuum-packaging of mackerel fillets was effective in mitigating lipid oxidation monitored as PV, TBARS as well as rancid odour and flavour, achieving a sensory shelf life of more than 10 months at − 25 °C, while that of the glazed fillets ended at 8 months. Use of shatter layer packaging provided a moderate protection against lipid oxidation and delayed the development of rancid odour and flavour by 2 months. The inclusion of seawater altered the sensory textural attributes of the mackerel fillet to be more juicy, tender and soft and increased the attribute of salty flavour in the VAC-S sample while masking rancid odour and flavour. Processing of mackerel including filleting, handling, double-freezing and glazing under industrial conditions increased the formation of FFA as well as the loss of WHC and cooking yield in the mackerel fillets regardless the packaging methods.
References
Aubourg SP, Gallardo JM (2005) Effect of brine freezing on the rancidity development during the frozen storage of small pelagic fish species. Eur Food Res Technol 220(2):107–112
Aubourg SP, Pineiro C, Gonzalez MJ (2004) Quality loss related to rancidity development during frozen storage of horse mackerel (Trachurus trachurus). J Am Oil Chem Soc 81(7):671–678
Aubourg SP, Ugliano M (2002) Effect of brine pre-treatment on lipid stability of frozen horse mackerel (Trachurus trachurus). Eur Food Res Technol 215(2):91–95
Aubourg SR, Rodriguez A, Gallardo JM (2005) Rancidity development during frozen storage of mackerel (Scomber scombrus): effect of catching season and commercial presentation. Eur J Lipid Sci Technol 107(5):316–323
Bernárdez M, Pastoriza L, Sampedro G, Herrera JJR, Cabo ML (2005) Modified method for the analysis of free fatty acids in fish. J Agric Food Chem 53(6):1903–1906
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(1):911–917
Christensen LB, Hovda MB, Rode TM (2017) Quality changes in high pressure processed cod, salmon and mackerel during storage. Food Control 72:90–96
Cyprian OO, Sveinsdottir K, Nguyen MV, Tomasson T, Thorkelsson G, Arason S (2017) Influence of lipid content and packaging methods on the quality of dried capelin (Mallotus villosus) during storage. J Food Sci Technol 54(2):293–302
Cropotova J, Mozuraityte R, Standal IB, Rustad T (2019) The influence of cooking parameters and chilled storage time on quality of sous-vide Atlantic mackerel (Scomber scombrus). J Aquat Food Prod Technol 28(5):505–518
Decker EA, Hultin HO (1990) Factors influencing catalysis of lipid oxidation by the soluble fraction of mackerel muscle. J Food Sci 55:947–950
Eide O, Børresen T, Strøm T (1982) Minced fish production from capelin (mallotus villosus) a new method for gutting, skinning and removal of fat from small fatty fish species. J Food Sci 47(2):347–349
Fjermestad A, Hemre GI, Holm JC, Totland GK, Froyland L (2000) Effects of different dietary fat levels in cage-fed Atlantic mackerel (Scomber scombrus). Eur J Lipid Sci Technol 102(4):282–286
Hashimoto K, Kawashima T, Yoshino N, Shirai T, Takiguchi A (2015) Effects of freshness on thawing drip and ice crystal formation in frozen spotted mackerel Scomber australasicus. Nippon Suisan GakkaishiGakkaishi 81(1):124–129
Hashimoto K, Kobayashi S, Yamashita M (2017) Comparison of connective tissue structure and muscle toughness of spotted mackerel Scomber australasicus and Pacific mackerel S. japonicus during chilled and frozen storage. Fish Sci 83(1):133–139
Huang X-H, Qi L-B, Fu B-S, Chen Z-H, Zhang Y-Y, Du M, Dong X-P, Zhu B-W, Qin L (2019) Flavor formation in different production steps during the processing of cold-smoked Spanish mackerel. Food Chem 286:241–249
Kanner J, Doll L (1991) Ferritin in turkey muscle tissue: a source of catalytic iron ions for lipid peroxidation. J Agric Food Chem 39(2):247–249
Lowry RR, Tinsley IJ (1976) Rapid colorimetric determination of free fatty acids. J Am Oil Chem Soc 53(7):470–472
Maestre R, Pazos M, Medina I (2011) Role of the raw composition of pelagic fish muscle on the development of lipid oxidation and rancidity during storage. J Agric Food Chem 59(11):6284–6291
Mariutti LRB, Bragagnolo N (2017) Influence of salt on lipid oxidation in meat and seafood products: a review. Food Res Int 94:90–100
Medina I, González MJ, Iglesias J, Hedges ND (2009) Effect of hydroxycinnamic acids on lipid oxidation and protein changes as well as water holding capacity in frozen minced horse mackerel white muscle. Food Chem 114(3):881–888
Pazos M, Maestre R, Gallardo JM, Medina I (2013) Proteomic evaluation of myofibrillar carbonylation in chilled fish mince and its inhibition by catechin. Food Chem 136(1):64–72
Pedrós-Garrido S, Condón-Abanto S, Beltrán JA, Lyng JG, Brunton NP, Bolton D, Whyte P (2017) Assessment of high intensity ultrasound for surface decontamination of salmon (S. salar), mackerel (S. scombrus), cod (G. morhua) and hake (M. merluccius) fillets, and its impact on fish quality. Innov Food Sci Emerg Technol 41:64–70
Petillo D, Hultin HO, Krzynowek J, Autio WR (1998) Kinetics of antioxidant loss in mackerel light and dark muscle. J Agric Food Chem 46(10):4128–4137
Popelka P, Luptakova O, Marcincak S, Nagy J, Mesarcova L, Nagyova A (2012) The effect of glaze and storage temperature on the quality of frozen mackerel fillets. Acta Veterinaria Brno 81(4):397–402
Richards MP, Hultin HO (2002) Contributions of blood and blood components to lipid oxidation in fish muscle. J Agric Food Chem 50(3):555–564
Romotowska PE, Gudjónsdóttir M, Karlsdóttir MG, Kristinsson HG, Arason S (2017) Stability of frozen Atlantic mackerel (Scomber scombrus) as affected by temperature abuse during transportation. LWT Food Sci Technol 83:275–282
Romotowska PE, Gudjónsdóttir M, Kristinsdóttir TB, Karlsdóttir MG, Arason S, Jónsson Á, Kristinsson HG (2016) Effect of brining and frozen storage on physicochemical properties of well-fed Atlantic mackerel (Scomber scombrus) intended for hot smoking and canning. LWT Food Sci Technol 72:199–205
Romotowska PE, Karlsdóttir MG, Gudjónsdóttir M, Kristinsson HG, Arason S (2016) Influence of feeding state and frozen storage temperature on the lipid stability of Atlantic mackerel (Scomber scombrus). Int J Food Sci Technol 51(7):1711–1720
Romotowska PE, Karlsdóttir MG, Gudjónsdóttir M, Kristinsson HG, Arason S (2016) Seasonal and geographical variation in chemical composition and lipid stability of Atlantic mackerel (Scomber scombrus) caught in Icelandic waters. J Food Compos Anal 49:9–18
Schmedes A, Hølmer CS (1989) A new thiobarbituric acid (TBA) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidation. J Am Oil Chem Soc 66(6):813–817
Simeonidou S, Govaris A, Vareltzis K (1997) Effect of frozen storage on the quality of whole fish and fillets of horse mackerel (Trachurus trachurus) and mediterranean hake (Merluccius mediterraneus). Zeitschrift für Lebensmitteluntersuchung und -Forschung A 204(6):405–410
Sone I, Skåra T, Olsen SH (2019) Factors influencing post-mortem quality, safety and storage stability of mackerel species: a review. Eur Food Res Technol 245(4):775–791
Standal IB, Mozuraityte R, Rustad T, Alinasabhematabadi L, Carlsson N-G, Undeland I (2018) Suality of filleted atlantic mackerel (scomber scombrus) during chilled and frozen storage: changes in lipids, vitamin d, proteins, and small metabolites, including biogenic amines. J Aquat Food Prod Technol 27(3):338–357
Stone H, Bleibaum RN, Thomas HA (2012) Chapter 6—descriptive analysis. In: Stone H, Bleibaum RN, Thomas HA (eds) Sensory evaluation practices, 4th edn. Academic Press, San Diego, pp 233–289
Tamotsu S, Sugita T, Tsuruda K, Fukuda Y, Kimura I (2012) Recovery from stress of spotted mackerel Scomber australasicus by briefly resting in a fish cage after capture stress treatment. Nippon Suisan GakkaishiGakkaishi 78(3):454–460
Thorarinsdottir KA, Gudmundsdottir G, Arason S, Thorkelsson G, Kristbergsson K (2004) Effects of added salt, phosphates, and proteins on the chemical and physicochemical characteristics of frozen cod (gadus morhua) fillets. J Food Sci 69(4):144–152
Tzikas Z, Papavergou E, Soultos N, Ambrosiadis I, Georgakis S (2009) Quality loss of Mediterranean Horse mackerel (Trachurus mediterraneus) skinned fillets kept under vacuum during frozen storage. J Aquat Food Prod Technol 18(3):266–283
Undeland I, Hultin HO, Richards MP (2002) Added triacylglycerols do not hasten hemoglobin-mediated lipid oxidation in washed minced cod muscle. J Agric Food Chem 50(23):6847–6853
Acknowledgements
Open Access funding provided by Nofima the food research institute. The authors would like to gratefully acknowledge the financial support of Nordic Innovation (MAR14306). Prof. María Guðjónsdóttir at University of Iceland is acknowledged for her valuable feedback on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
The work does not contain any studies with human participants or living animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sone, I., Sveinsdóttir, H.I., Stefánsson, G. et al. Investigating commercially relevant packaging solutions to improve storage stability of mechanically filleted Atlantic mackerel (Scomber scombrus) produced under industrial conditions. Eur Food Res Technol 246, 693–701 (2020). https://doi.org/10.1007/s00217-020-03434-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03434-x